
Concept explainers
For each pair of ions, determine which will have the greater number of unpaired electrons: (a) Fe2+, Fe3+; (b) P3+, P5+: (c) Cr2+, Cr3+.
(a)
Interpretation: Ground-state electronic configuration of the given set of ions has to be written.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this valence orbital diagram can be drawn for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions, the electronic configuration is written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To identify: The ion which has greater number of unpaired electrons.
Answer to Problem 4.78QP
Answer
In (a)
Explanation of Solution
Electronic configuration of
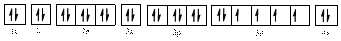
The electronic configuration of
Electronic configuration of
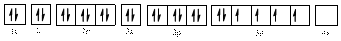
The electronic configuration of
Electronic configuration of
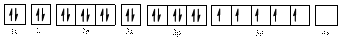
The electronic configuration of
Comparing the unpaired electrons in

By looking at the valence-orbital diagram we can see that in
(b)
Interpretation: Ground-state electronic configuration of the given set of ions has to be written.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this valence orbital diagram can be drawn for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions, the electronic configuration is written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To identify: The ion which has greater number of unpaired electrons.
Answer to Problem 4.78QP
Answer
In (b)
Explanation of Solution
Electronic configuration of
The electronic configuration of
Electronic configuration of
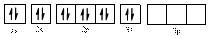
The electronic configuration of
Electronic configuration of
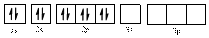
The electronic configuration of
Comparing the unpaired electrons in
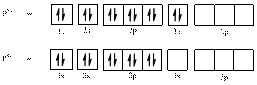
By looking at the valence-orbital diagram we can see that both electrons in
(c)
Interpretation: Ground-state electronic configuration of the given set of ions has to be written.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this valence orbital diagram can be drawn for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions, the electronic configuration is written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To identify: The ion which has greater number of unpaired electrons.
Answer to Problem 4.78QP
Answer
In (c)
Explanation of Solution
Electronic configuration of
The electronic configuration of
Electronic configuration of
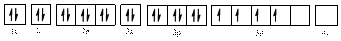
The electronic configuration of
Electronic configuration of
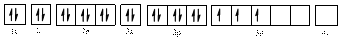
The electronic configuration of
Comparing the unpaired electrons in

By looking at the valence-orbital diagram we can see that in
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: Atoms First
- Write condensed electron configurations for the following: (a) Zr; (b) V3+; (c) Mo3+.arrow_forwardUse the periodic table to (i) predict electron configurations for the following species: Arsenic ion, As3– Magnesium ion, Mg2+ Vanadium(II) ion, V2+ (ii) Write the electron configurations of each species in the noble gas notation. (iii) Draw an orbital diagram to represent 1 c above. Draw the Lewis electron dot structures of the following chemical species. In each case you must say whether or not the central atom obeys the Octet Rule. CS2 and H2S CF4 and SiH4 NH2Cl CO32– and BF3 PCl5 ClF3, XeF2, Calculate the formal charge on the Sulphur atom in the Sulphate anion structure shown below(picture attatched) Give the electron-pair and molecular geometries for NF3 and XeF4.arrow_forwardWrite the charge and full ground-state electron configuration of the monatomic ion most likely to be formed from each atom: (a) CI (b) Na (c) Ca Which of the formed ions will be least stable? -- --arrow_forward
- Draw Lewis structures for the following species: Br2Se, NCCN, GeO4 4- , HO2 - , NBr4 + . Write down the condensed electron configuration of the following ions: a) Fe+ b) Zn c) Ga2+ d) Cu3+arrow_forward11)Explain the given ionization energy for each pair using electron configurations. (12(Be)l1(B), I1(N)>l1(O) ).arrow_forwardWrite the electron configuration for the monatomic ions formed from the following elements (which form the greatest concentration of monatomic ions in seawater):(a) Cl(b) Na(c) Mg(d) Ca(e) K(f) Br(g) Sr(h) Farrow_forward
- Which of the species NP, NP+ and NP2− would be paramagnetic? Briefly explain why (one sentence).arrow_forward(a) Which of the following atoms or ions is diamagnetic?Cu2+ Se4+ V2+ Co3+ V4+ (b) Which of the following atoms or ions is paramagnetic?Cl3+ N3+ Mn7+ V5+ Nearrow_forwardWrite electron configurations for the following ions, anddetermine which have noble-gas configurations: (a) Cd2+,(b) P3-, (c) Zr4+, (d) Ru3+, (e) As3-, (f) Ag+.arrow_forward
- 1- Give the electron configurations for the following ions? Al * (Z=13) Fe* (Z=26) and 2- Calculate the number of covalent bonds of Fluorine (F), Z of F equal (9)?arrow_forwardWrite the ground-state electron configuration for each atomand ion pair-Zr, Zr2+, Co, Co2+, Tc, Tc3+, Os, Os4+?arrow_forwardUse the noble-gas notation and write the ground-state electronic configurations of the following ions:(a) Ca2+ (b) Ga3+ (c) Cr3+arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





