
Concept explainers
(a)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(a)
Explanation of Solution
Given molecule,
Increase in
(b)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(b)
Explanation of Solution
Given molecule,
Increase in
(c)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(c)
Explanation of Solution
Given molecule,
Increase in
(d)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(d)
Explanation of Solution
Given molecule,
Increase in
(e)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(e)
Explanation of Solution
Given molecule,
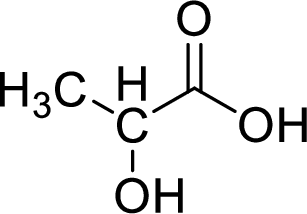
Increase in
(f)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(f)
Explanation of Solution
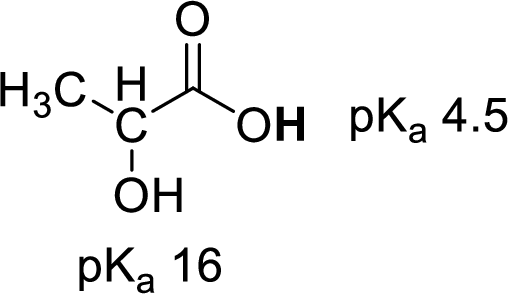
Given molecule,
Increase in
(g)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(g)
Explanation of Solution
Given molecule,
Increase in
(h)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(h)
Explanation of Solution
Given molecule,
Increase in
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
- Of the following carboxylic acids, which is the most acidic? (A) CH3CO₂H (B) HCO₂H (C) CO₂H (D) Cl₂CCO₂H (E) (CH3)3CCO₂Harrow_forwardWhich compound in each pair is more basic: (a) (CH3)2NH and NH3; (b) CH3CH2NH2 and ClCH2CH2NH2?arrow_forward(i) Draw the dissociation reaction for a carboxylic acid in water and define the Ka for this reaction. Write the equation that relates pKa to Ka. (ii) A deprotonated carboxylic acid can be drawn in two resonance forms. Draw the two forms and explain what the term “resonance” means. (iii) Draw an energy profile for the above dissociation reaction and describe how the profiles for a strong and a weak acid would differ.arrow_forward
- 1.(a) Which of the following groups has the LOWEST IUPAC priority?(A) CH3 (B) NH2 (C) OH (D) COOH (E) Br (b)Which of the following corresponds to the strongest acid?(A) (CF3)3C-COOH (B) (CF3)3 C-OH(C) CH3COOH (D) CH3OH(E) HOCH2CH3arrow_forward2-35 Predict which compound in each pair has the higher boiling point. Explain your prediction. (a) CH3CH₂OCH3 or CH3CH(OH)CH3 (b) CH3CH₂CH₂CH3 or CH3CH₂CH₂CH₂CH3 (d) CH3CH₂CH₂CH₂CH3 or CH3CH₂CH₂CH₂CH₂Cl (c) CH3CH₂CH₂2CH₂CH3 or (CH3)2CHCH₂CH3 CH3 ΝΗ or CH₂ NH or " NHarrow_forwardThe enamine prepared from acetone and dimethylamine is shown here in its lowest-energy form. (a) What is the geometry and hybridization of the nitrogen atom? (b) What orbital on nitrogen holds the lone pair of electrons? (c) What is the geometric relationship between the p orbitals of the double bond and the nitrogen orbital that holds the lone pair? Why do you think this geometry represents the minimum energy?arrow_forward
- Nepheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a) Label the most acidic H atom. (b) Which carbon–carbon σ bond is shortest? (c) How many degrees of unsaturation does nepheliosyne B contain? (d) How many bonds are formed from Csp–Csp3? (e) Label each triple bond as internal or terminal.arrow_forwardThe pka of phenol (C6H5OH) is 10.0. When a nitro group (NO2) is attached to the ring, the pK, decreases, as shown for the ortho, meta, and para isomers. OH OH ОН OH NO2 (a) Explain why the pK, values of all three isomers are lower than the pka of phenol itself. (b) Explain why the meta isomer has the highest pka of `NO2 Phenol NO2 the three isomers. pKa = 10.0 7.23 8.35 7.14arrow_forwardно HO но он The pK, of ascorbic acid (vitamin C) is 4.17, showing that it is slightly more acidic than acetic acid (CH3CO0H, pKa 4.74). (a) Show the fou r different conjugate bases that would be formed by deprotonation of the four different OH groups in ascorbic acid. (b) Compare the stabilities of these four conjugate bases, and predict which OH group of ascorbic acid is the most acidic. (c) Compare the most stable conjugate base of ascorbic acid with the conjugate base of acetic acid, and suggest why these two compounds have similar acidities, even though ascorbic acid lacks the carboxylic acid (COOH) group.arrow_forward
- (a) (b) CH3 *3 CH3 CH3 TAYY OH -85 H₂ / Pt n [ ]# H₂/ Pt ? ChemDoodleⓇarrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forwardPredict the products of the following acid-base reactions. If the equilibrium would not result in the formation of appreciable amounts of products, you should so indicate. In each case label the stronger acid, the stronger base, the weaker acid, and the weaker base: (a) CH3CH=CH2 + NANH2 (d) CH3C=C: + CH;CH2OH → (e) CH3C=C:- + NH¾CI – | (b) CH;C=CH + NaNH2 (c) CH3CH2CH3 + NANH2 → | HASarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





