
(a)
Interpretation:
The standard free energy of activation of reaction B is to be calculated.
Concept introduction:
The rate of the reaction is affected by the activation free energy of the reaction. The relationship between the activation free energy and rate of reaction is given by,
Where,
•
•
•
Answer to Problem 4.29P
The standard free energy of activation for reaction B is
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The relative rates of two reactions are expressed as,
Where,
•
•
•
Substitute the activation energy for reaction A, the relative rate of A and B, gas constant and temperature in the given formula.
Rearrange the above equation for the calculation of activation energy of B as shown below.
Thus, standard free energy of activation for reaction B is
The standard free energy of activation for reaction B is
(b)
Interpretation:
The reaction free energy diagram for the two reactions showing the two values of
Concept introduction:
The transition state is formed during the conversion of reactants into products in the
Answer to Problem 4.29P
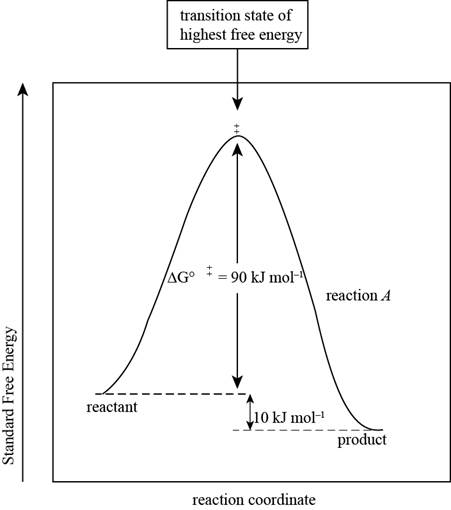
The reaction free-energy diagram for the reaction A showing the two values of
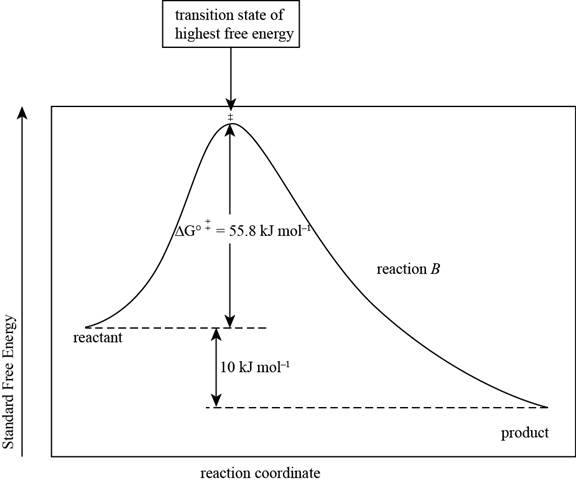
The reaction free-energy diagram for the reaction B showing the two values of
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The reaction free-energy diagram for the reaction A showing the two values of
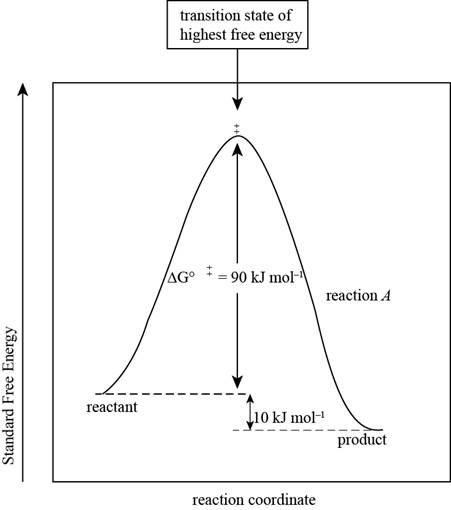
Figure 1
This diagram represents the plot between standard free energy and the reaction coordinates. The point at which the energy is maximum represents the transition state of the reaction.
The reaction free energy diagram for the reaction B showing the two values of
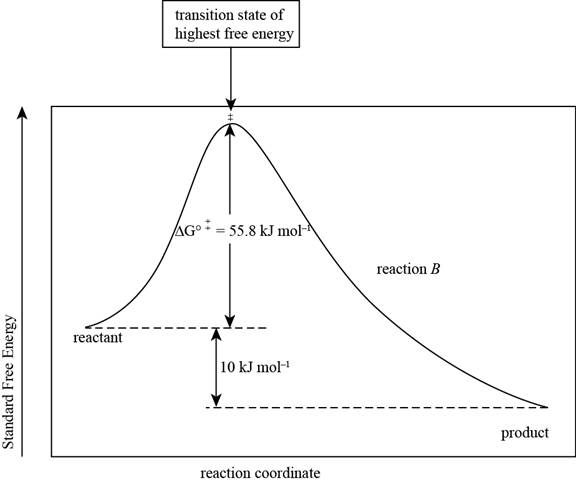
Figure 2
The reaction free-energy diagram for the two reactions showing the two values of
(c)
Interpretation:
The standard free energy of activation of the reverse reaction in both reactions A and B is to be calculated.
Concept introduction:
The free energy diagram represents the plot between standard free energy and the reaction coordinates. The point at which the energy is maximum represents the transition state of the reaction.
Answer to Problem 4.29P
The standard free energy of activation of the reverse reaction in both reactions A and B is
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The products of each reaction are
The standard free energy of activation of the reverse reaction A is,
Thus, the standard free energy of activation of the reverse reaction A is
The standard free energy of activation of the reverse reaction B is,
Thus, the standard free energy of activation of the reverse reaction A is
The standard free energy of activation of the reverse reaction in both reactions A and B is
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
- 2b which determines whether a reaction is spontaneous. the net free energy change of the reaction or the activation energy of the reactionarrow_forwardCalculate the equilibrium constant for the phosphorylation of glucose to glucose 6-phosphate at 37.0 °C. 4.74 x10¬3 M-1 eq In the rat hepatocyte, the physiological concentrations of glucose and P; are maintained at approximately 4.8 mM. What is the equilibrium concentration of glucose 6-phosphate (G6P) obtained by the direct phosphorylation of glucose by P;? 8.75 x10-8 [G6P] = M Incorrectarrow_forwardThe reaction glucose + phosphate ⇔ glucose-6-phosphate + H2O has ΔG° = 13.8 kJ mol-1. Calculate ΔGrxn in kJ mol-1 at 37.0 °C when [glucose] = 19.0 mmol L-1, [phosphate] = 6.90 mmol L-1, [glucose-6-phosphate] = 1.70 mmol L-1, and [H2O] = 55.5 mol L-1. (R = 8.3145 J mol-1 K-1)arrow_forward
- For the reaction CO(NH2)2 +H2O(l) -> CO2(g) + 2NH3(g) The ∆S^2 and ∆H^2 is 354.8KJ/mol and 119.2KJ/mol respectively. Calculate (a) the standard entropy of urea(b) the standard enthalpy of formation of urea(c) the standard free energy change of the reaction and (d) the standard free energy of formation of urea.arrow_forwardThe conversion of n-butane to 2-methylpropane is an equilibrium process with ΔH° = −2.05 kcal/mol and ΔG° = −0.89 kcal/mol at 298 K. What is the change in entropy for this conversion?arrow_forwardIn glycolysis, the hydrolysis of ATP to ADP is used to drive the phosphorylation of glucose (GLC): GLC+ATP <--> ADP+GLC-6-phosphat ΔG° = −17.7 kJ What is the value of Kc for this reaction at 298K?arrow_forward
- A reaction has a standard free‑energy change of −14.80 kJ mol−1(−3.537 kcal mol−1). Calculate the equilibrium constant for the reaction at 25 °C.arrow_forwardCalculate AH for the reaction C(graphite) + 2H2(9) → CHạ using the following data: C(graphite) + O2(g) → CO2(9) AH = -393.5 kJ H2«9) + 02c9) → H20m H201) AH = -285.8 kJ ΔΗ- CH49) + 202(9) → CO2(g) + 2H201) AH = -890.4 kJarrow_forwardCalculate ΔG in kJ•mol-1 at 298 K for the following reaction: 2 A(g) + B2(s) → 2 AB(g) ΔGo = 49 kJ•mol-1 under the following conditions: P(A) = 0.446 atm, P(AB) = 0.823 atm A certain reaction A → B has a rate constant of 4.7 x 10–2 M s-1 at 25°C. An experiment was run at this temperature where the initial concentration of A is 0.342 M. What is the half-life of the reaction in seconds? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3)arrow_forward
- Using the thermodynamic informationn, claculate the standard reaction free energy of the following chemical reaction: Fe2O3(s)+3H2(g)---> 2Fe(s)+3H2O(l)arrow_forward(a) The equilibrium constant of the isomerization of cis-2-butene to trans-2-butene is 2.07 at 127.0 °C. Determine the standard molar Gibbs free energy, AGR º for the reaction. (b) For C6o, the standard molar Gibbs free energy (AG, º) of formation from the elemental carbon is 23.98 kJ/mol at 25.0 °C. Determine the equilibrium constant, Keq for the formation of C60.arrow_forwardP17B.9 The gas phase decomposition of ethanoic acid at 1189K proceeds by way of two parallel reactions: k, = 3.74s k, = 4.65s (1) CH,COOH → CH, + CO, (2) CH,COOH → CH,CO+ H,O (a) What is the maximum theoretical yield of the ketene CH,CO at this temperature? (b) Does the ratio of ketene to methane vary over time?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
