
Interpretation:
The
Concept introduction:
The anti and gauche bonds in cyclohexane are represented by sawhorse projections of staggered conformations of
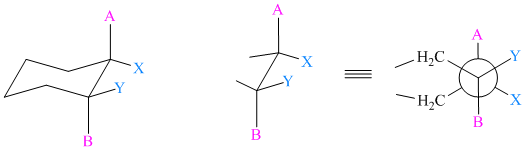
The substituents in anti-relationship are
The substituents in gauche relationship are
In the following structure, each path shows anti relationship where a substituent is equatorial.
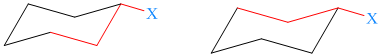
In the following structure, each path shows gauche relationship where a substituent is axial.

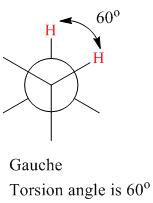
The torsion (dihedral) angle for eclipsed conformation is

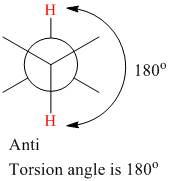
The torsion (dihedral) angle for gauche conformation is
The torsion (dihedral) angle for anti-conformation is
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry - Standalone book
- Tell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated. (a) cis-1, 3-Dibromocyclohexane and trans-1, 4-dibromocyclohexane (b) 2, 3-Dimethylhexane and 2, 3, 3-trimethy1pentanearrow_forwardFollowing is a chair conformation of cyclohexane with the carbon atoms numbered 1 through 6. (a) Draw hydrogen atoms that are above the plane of the ring on carbons 1 and 2 and below the plane of the ring on carbon 4. (b) Which of these hydrogens are equatorial? Which are axial? (c) Draw the alternative chair conformation. Which hydrogens are equatorial? Which are axial? Which are above the plane of the ring? Which are below it?arrow_forwardIdentify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):arrow_forward
- Construct a qualitative potential energy diagram for rotation about C-C bond of 1,2-dibromoethan. Which conformation would you expect to be more stable? label the anti and gauche conformation of 1,2-dibromoethanearrow_forwardDII F3 Provide the correct IUPAC name for the compound shown here. x F4 4, F5 O= CI-C-CH₂-CH₂-CH3 Question 8 of 20 2- 5- tert- 小 6- F6 () 3- sec- tri iso cyclo di chloro eth pent but prop chlor OIC acid oyl yl an ide 4- F7 W PrtScn F8 Home 4 F9 End F10 PgUparrow_forwardDraw the most stable conformation for each of the following substituted cyclohexanes. Show all conformation in Newman projections. Fill in hydrogens to indicate unsubstituted carbons.arrow_forward
- Use valence-shell electron-pair repulsion (VSEPR) model to predict the bond angle for each of the following highlighted carbon atoms. (a) (b) -CH2OH (c) HC c- -CH=CH2 (d) (a) red carbon blue carbon (b) red carbon (c) red carbon blue carbon (d) red carbonarrow_forwardConsidering rotation around the bond highlighted in red, draw the Newman projection for the most stable and least stable conformations when viewed down the red bond in the direction of the arrow. H₂C Part 1 of 2 H H CH3 4 Draw the Newman projection for the most stable conformation.arrow_forwardCH;CH3 Br CH3 If you take the above compound, and place an ISOPROPYL group on the carbon of the ring directly between the bromo and methyl groups, you will have a ring with four groups, all on different neighboring carbons. The correct IUPAC name of this new compound would be (including numbers and proper punctuation when required):arrow_forward
- For rotation about the C-a – C-b bond, using the following templates, draw the Newman Projection of the two staggered conformers that are different in energy. Fill each bond on Newman Projection with Hydrogen or Substituents. You may use Abbreviations such as Me for Methyl, Et for Ethyl, iPr for isopropyl. Circle the Newman projection of the most stable conformer.arrow_forwardpoint the IUPAC name for the following structure CH3CH2CHBrCH=C(CH2CH3)2 3-ethyl-5-bromohept-3-ene O 5-bromo-3-ethylhept-3-ene O 3-bromo-5-ethylhept-4-ene 1,1-diethyl-3-bromopent-1-ene O Option 5 Oarrow_forwardConsider the molecule shown below. NH2 Он Which of the following statements is true regarding this structure? O In the most stable chair conformer, both the amine and the cyclopentyl substituents would be in the equatorial position. O In the most stable chair conformer, both the amine and the alcohol substituents would be in the equatorial position. O The most stable chair conformer has no steric strain. O The cyclopentane ring is planar. O The alcohol substituent is trans to the amine substituent.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

