
Concept explainers
(a)
Show the cubic direction
(a)
Explanation of Solution
Show the cubic direction
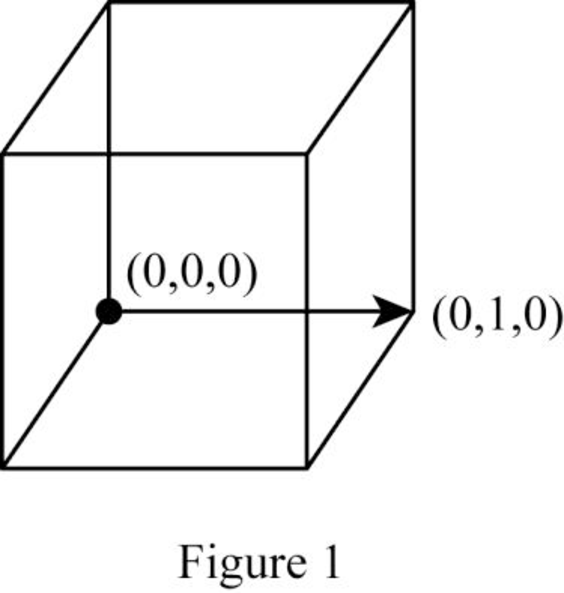
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(b)
Show the cubic direction
(b)
Explanation of Solution
Show the cubic direction
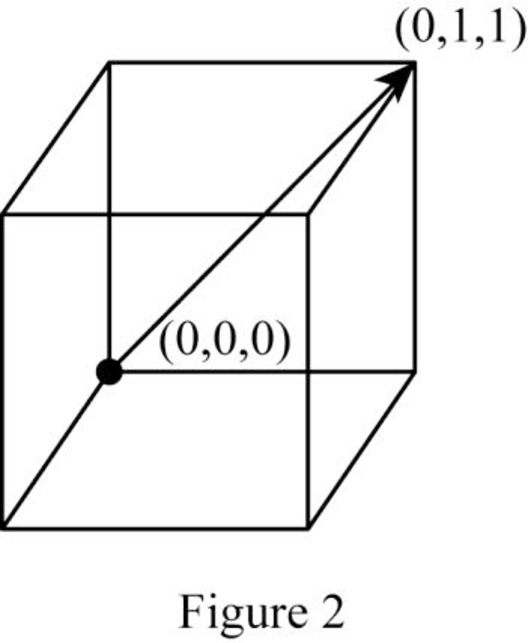
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(c)
Show the cubic direction
(c)
Explanation of Solution
Show the cubic direction
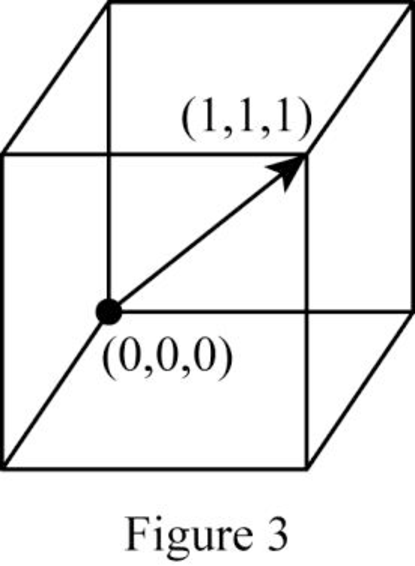
Position coordinates:
For the cubic direction
Repeat distance:
For the cubic direction
(d)
The angle between the cubic directions
(d)
Answer to Problem 29AAP
The angle between the cubic directions
Explanation of Solution
Write the expression to calculate angle between the cubic directions
Here, Miller indices of the cubic plane 1 are
Conclusion:
Substitute 0 for
Thus, the angle between the cubic directions
Want to see more full solutions like this?
Chapter 3 Solutions
Foundations of Materials Science and Engineering
- Determine the indices for the directions shown in the following cubic unit cell. Part 1 Z We begin with Direction A. (a) What is the x coordinate of the vector tail (x₁)? (b) What is the x coordinate of the vector head (x2)? (c) What is the y coordinate of the vector tail (y₁)? (d) What is the y coordinate of the vector head (y2)? (e) What is the z coordinate of the vector tail (2₁)? (f) What is the z coordinate of the vector head (z₂)? Fractional entries should be expressed in decimal form. H +yarrow_forward1. Within a cubic unit cell, sketch the following directions: [110], [121], [0Ī2], [123], [103]arrow_forward6. Within a cubic unit cell, sketch the following directions: ( :)) (a) [110] (e) [771] (b) [121] (f) [722] (c) [072] (d) [133] (g) [123] (h) [103]arrow_forward
- Draw the following direction vectors in an orthorhombic unitcell:[111],[201]and [2 ̄31]. with lattice parameters {1, 2, 3, 90, 90, 90}arrow_forward(b) Draw the following direction vectors in a cubic unit cell: [212] and [101] Draw the following crystallographic planes in a cubic unit cell: (111) and (121) "oin 2/2 Page 1 of 2arrow_forwardB) Draw the following planes in the hexagonal crystal: (1) (1 0i ) (2) (ī010) (3) ( 0ī 11) (4) ( 0 1 o) C) Draw the following direction vectors in the cubic unit cell: (1) [111] (2) [011] (3) [110] (4) [112]arrow_forward
- (a) Derive linear density expressions for BCC [110] and [111] directions in terms of the atomic radius R and (b) compute linear density values for these same two directions for tungsten. (a) [110]: i [111]: i (b) [110]: i [111]: eTextbook and Media atom/R atom/R 1/m 1/marrow_forwardWithin a cubic unit cell, sketch the following directions: (a) [110] (b) [121] (c) [012] (d) [133]arrow_forwardwithin a orthorhombic unit cell, sketch the following: [123] and [133]arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





