
(a)
Interpretation:
The critical temperature and critical pressure of a gasneeds to be defined.
Concept introduction:
Critical temperature is the temperature at which the vapor of the substance cannot be liquefied even if the pressure is applied. Critical pressure is the pressure at which the gas can be liquefied at its critical temperature.
Answer to Problem 29.1QAP
The temperature at which a substance cannot be liquefied from its vapor form is termed as critical temperature. The pressure at which the gas can be liquefied at its critical temperature is termed as critical pressure.
Explanation of Solution
When gases are compressed at suitable temperature, it gets converted to liquids. If the temperature is increased, the kinetic energy of the gas molecules increases making it more difficult for the gas to liquefy. Even if the pressure is increased, it does not have an impact. Hence, this temperature at which the gas cannot be liquefied is termed as critical temperature.
Similarly, the pressure that is required to liquefy a gas at its critical temperature, is said to be critical pressure.
(b)
Interpretation:
Supercritical fluid needs to be defined.
Concept introduction:
A fluid that does not exist as liquid or gas above its critical temperature and pressure is said to be supercritical fluid. The special features of this fluid is that it can pass through solid like gas and dissolve materials as liquid does.
Answer to Problem 29.1QAP
Supercritical fluids are those that exist having their temperature and pressure beyond the critical point. Hence, it has low viscosity and high density. This does not allow for the substance to liquefy even if the pressure is increased.
Explanation of Solution
Fluids that does not have a distinct liquid or gaseous phase above its critical point is termed as supercritical fluid.
Upon observing the graph, it can be noticed that above the critical point, the substance cannot exist as a liquid or gas.
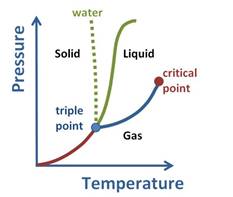
This is due to the fact that at higher temperatures, the kinetic energy of the molecules is high which will help overcome the intermolecular forces that are necessary for bringing about condensation or converting to liquid phase.
Besides this, at a high pressure, or pressure above critical pressure the substance cannot stay in gaseous state. There needs to be a balance between the two states.
Hence, the state at which the substance does not exist as liquid or gas is said to be supercritical fluid. It behaves such that it can pass through solid as gas does and dissolve materials as liquid does. This property makes it suitable as substitute for organic solvents. In other words, supercritical fluids behave as gas and as liquid.
Want to see more full solutions like this?
- Region x is (liquid, Supercritical Fluid, Solid, Gas) Region w is (liquid, Supercritical Fluid, Solid, Gas) Region y is (liquid, Supercritical Fluid, Solid, Gas) Point z is (liquid, Supercritical Fluid, Solid, Gas)arrow_forward9. The critical temperature of oxygen is 155 K. What does this mean? (a) Oxygen is very cold. (b) The critical pressure must be > 155 bar. (c) It is impossible to produce liquid oxygen by compression alone if its temperature is > 155 K. (d) It is critical to keep oxygen at a temperature of 155 K.arrow_forwardA gas mixture at room temperature contains 10.0 mol CO and 12.5 mol 0,. (a) Compute the mole fraction of CO in the mixture. (b) The mixture is then heated, and the CÓ starts to react with the O, to give CO2: CO(g) + O2(g) –→ CO2(g) At a certain point in the heating, 3.0 mol CO, is present. Determine the mole fraction of CO in the new mixture.arrow_forward
- Pressure (A) Explain the term pressure and state its S.I. unit. (B) Explain Henry’s law. (C)A bottle of H2 has just been received by the technicians in DkIT for use in the instrumentation lab. It is a 47 litre cylinder at a pressure of 50 atmospheres. The normal working pressures is 2 bar. (i) To what volume of gas will that equate at the working pressure? (ii) For how many hours will the gas last if it used at the rate of 0.5dm3 per hour? D)You see your best friend at the bar and you walk up behind her. You accidentally startle her and she takes a step backwards. Unfortunately, she is wearing high heels and her heels come down on your foot. She weighs only 55kg but the size of her heel is 6mm by 6mm.Determine the pressure that she applies on your foot. (E) If a diver dives to a depth of 35 m what will be: (i) the pressure in Pascals due the water column? (3 marks) (ii) the pressure of the air in…arrow_forward2. A sample of vapor weighing 0.368 g filled a Florence flask when heated in a water bath at 95.0°C. The actual volume of the flask was 128.4 mL and the barometric pressure was 747 torr. Calculate (a) the volume of the vapor at STP and (b) the molar mass of the volatile liquid. (Use the approach applied in the Introduction and on the data sheet.)arrow_forwardQ23(i) Write any two applications of Kinetic Theory of gases.arrow_forward
- a) From the temperature-pressure data graphed in Part A, visually determine the gas pressure at a temperature of 350 K, including units. (b)From the temperature-pressure data graphed in Part A, determine the equation of the line of best fit. (C) What are the units for the slope of the line of best fit determined in question 2? (D) From the equation of the line of best fit determined in Question 2, algebraically determine the gas pressure at a temperature of 350 K, including units and a unit analysis. Note: I only need su part D solve pleasearrow_forwardWhat is the respiration rate for a breath every 2.5 seconds? What solids are made up of large numbers of independent, constituent units, some of which attract and others repel.arrow_forwardWhat is the temperature in K of 0.500 mole of neon in a 2.00L vessel at 4.32 bar? R = 0.08314 L· bar/mol·K.arrow_forward
- A 1.52 g mixture of sucrose (CH,0, 342.30 g mol") and ethyl alcohol (C,H,O, 46.07 g mol") is reacted with acidic aqueous potassium dichromate (K,Cr,0,) solution. Produced 3.12 L CO, (g) is collected over water at 35 °C and has a barometric pressure of 0.52 atm. Water has a vapor pressure of 42.20 mmHg. Calculate the mass percent of C,H,O, in the mixture. CHOlaq) + Cr,O, (aq) - CO.(g) + Cr"(aq) (not balanced) CH.O(aq) + Cr,0, (aq) - CO.(g) + Cr" (aq) (not balanced) Enter only integer number, do not use % sign, Le. 12.345% should be entered as 12. Answer:arrow_forwardDescribe what happens to the average kinetic energy of ideal gas molecules when the conditions are changed as follows:(a) The pressure of the gas is increased by reducing the volume at constant temperature.(b) The pressure of the gas is increased by increasing the temperature at constant volume.(c) The average velocity of the molecules is increased by a factor of 2.arrow_forwardNatural gas is a mixture of hydrocarbons, primarily methane (CH4) and ethane (C2H6). A typical mixture might have mole fraction of methane = 0.915 and mole fraction of ethane = 0.085. (a) What are the partial pressure of the two gases in a 14.10 L container of natural gas at 30 degrees Celcius and 1.34 atm? (b) Assuming complete combustion of both gases in the natural gas sample, what is the total mass of water formed? Hint: Write the balanced combustion chemical equation for each gas separately in the gas mixture to find the mass of water formed.arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

