
Concept explainers
Interpretation:
The amounts, in milligrams, of
Concept Introduction:
In an empirical formula, the mass of each element changes into moles by taking the molar mass from the periodic table.
Each mole value is divided by the smallest number of calculated moles.
Answer to Problem 100AP
Solution:
(a) The amounts of
(b)
(c)
Explanation of Solution
Given information: The mass of compound Y =
The mass of
The mass of
a)The mass of
The mass of
The mass of
The mass of oxygen is calculated as follows:
b)Derive the empirical formula of
The calculated value of compound
The mass of each element changes into moles by taking the molar mass from the periodic table as follows:
Each mole is divided by the smallest number of calculated moles and taken round to the nearest whole number.
The value of each element is considered as the mole ratio of the elements, which is represented by subscripts in the empirical formula, as follows:
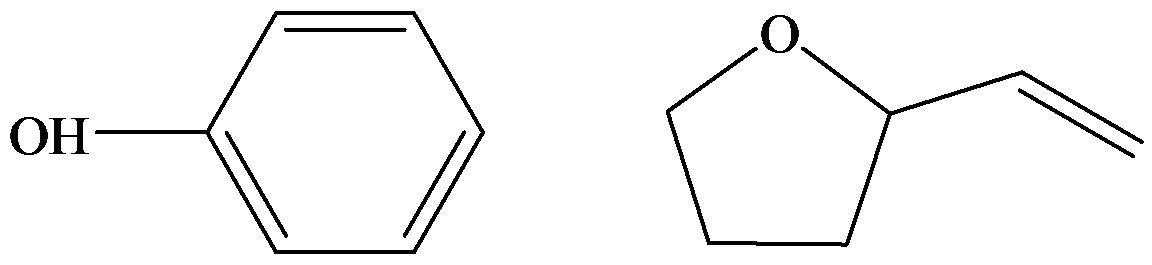
c) Plausible structure for
The compound
The structures are represented below.
Want to see more full solutions like this?
Chapter 25 Solutions
Chemistry
- Illustrate the chemical structural formula for 3-methyl-3-ethylpentane. (b) Identify its chemical family as an isomer. (c) Provide the balanced chemical reaction equation for the combustion of one mole of this fuel with an equivalence ratio of ϕ=0.735arrow_forwardPropane, C3H8, is a hydrocarbon that is commonly used as a fuel.(a) Write a balanced equation for the complete combustion of propane gas.(b) Calculate the volume of air at 25 °C and 1.00 atmosphere that is needed to completely combust 25.0 grams of propane. Assume that air is 21.0 percent O2 by volume. (Hint:use the information that 1.00 L of air at 25 °C and 1.00 atm contains 0.275 g of O2 per liter.)(c) The heat of combustion of propane is −2,219.2 kJ/mol. Calculate the heat of formation, ΔH°f of propane given that ΔH°f of H2O(l) = −285.8 kJ/mol and ΔH°f of CO2(g) = −393.5 kJ/mol. (d) Assuming that all of the heat released in burning 25.0 grams of propane is transferred to 4.00 kilograms of water, calculate the increase in temperature of the water.arrow_forward(a) When a compound containing C, H, and O is completelycombusted in air, what reactant besides the hydrocarbonis involved in the reaction? (b) What products form in thisreaction? (c) What is the sum of the coefficients in the balancedchemical equation for the combustion of one mole ofacetone, C3H6O1l2, in air?arrow_forward
- This question is about the chemistry of alkenes, which are unsaturated hydrocarbons. (a) State what is meant by the term unsaturated as applied to a hydrocarbon. (1) (b) An organic compound, X, is an unsaturated hydrocarbon with molecular formula CH₂. (i) Draw the displayed formulae and give the names of two molecules with molecular formula C₂H, which are E/Z isomers. (3) Isomer 1 Isomer 2 Name: Name:arrow_forward(a) What structural feature is associated with each type of hydrocarbon: alkane, cycloalkane, alkene, and alkyne?(b) Give the general formula for each type.(c) Which hydrocarbons are considered saturated?arrow_forward(a) Calculate the standard enthalpy change for the combustion of 1 mol of benzene, C6H61l2, to CO21g2 and H2O1l2.(b) Compare the quantity of heat produced by combustion of 1.00 g propane with that produced by 1.00 g benzene.arrow_forward
- A certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon: (d) It contains an alkyne O It contains one ring and one double bond (c) It contains two double bonds and no rings O (b) It contains one ring and no double bondsarrow_forwardWrite the chemical formula and Lewis structure of the following, each of which contains five carbon atoms:(a) an alkane(b) an alkene(c) an alkynearrow_forwardWhich chemical formulas represent organic compounds and which represent inorganic compounds: (a) H 2SO 4; (b) Br 2; (c) C 5H 12?arrow_forward
- Alcohols A, B, and C all have the composition C4H10O. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde; molecules of alcohol B contain a linear carbon chain and can be oxidizedto a ketone; and molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone. Write the Lewis structures of these molecules.arrow_forwardExplain each statement in terms of atomic properties:(a) Carbon engages in covalent rather than ionic bonding.(b) Carbon has four bonds in all its organic compounds.(c) Carbon forms neither stable cations, like many metals, norstable anions, like many nonmetals.(d) Carbon bonds to itself more extensively than does any otherelement.(e) Carbon forms stable multiple bondsarrow_forward1. The structure of compound A is shown below. он NH2 (a) Redraw the above structure in the form of expanded and condensed structures. (b) Determine the number of primary, secondary, tertiary and quaternary carbon atom that can be found in the compound. (c) Redraw, circle and name the functional groups present in the compound. (d) State the possible type of stereoisomerism of the compound and draw the appropriate structures to describe the isomerism.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


