
Concept explainers
Name the following
(a) CH3CH2CH2NH2
(b) (CH3)3N
(c) (CH3)(C2H5)NH
(d) C6H13NH2
(a)
Interpretation: The name of the following amine has to be written.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
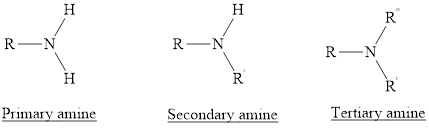
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Answer to Problem 40PS
The systematic name of the given amine,
Explanation of Solution
The molecular formula of the given amine is
It is a primary amine
One propyl
Therefore, the name of the given amine is propylamine.
(b)
Interpretation: The name of the following amine has to be written.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Answer to Problem 40PS
The systematic name of the given amine,
Explanation of Solution
The molecular formula of the given amine is
Three methyl
It is a tertiary amine
Therefore,
The name of the given amine is N,N-trimethylamine.
(c)
Interpretation: The name of the following amine has to be written.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Answer to Problem 40PS
The systematic name of the given amine,
Explanation of Solution
The molecular formula of the given amine is
One methyl
It is a secondary amine
Therefore, the name of the given amine is N-ethylmethylamine.
(d)
Interpretation: The name of the following amine has to be written.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Answer to Problem 40PS
The systematic name of the given amine,
Explanation of Solution
The molecular formula of the given amine is
It is a primary amine
One hexyl
Therefore the name of the given amine is hexylamine.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity
- Classify each compound (a)–(f) as one of the following: (i) amide, (ii) ester, or(iii) carboxylic acid. (a) CH3COOCH3 (b) RCONHR (c) C6H5COOHarrow_forwardGive the chemical tests to distinguish between the following pairs of compounds :(i) Ethyl amine and Aniline(ii) Aniline and Benzylaminearrow_forwardDraw structures corresponding to these names:(a) 4-Methylpentanamide (b) N-Ethyl-N-methylpropanamidearrow_forward
- Identify which of the statements is/are correct. (i) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (j) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forwardIndicate whether each statement is true or false: (a) Fat molecules contain amide bonds. (b) Phosphoplipids can be zwitterions. (c) Phospholipids form bilayers in water in order to have their long hydrophobic tails interact favorably with each other, leaving their polar heads to the aqueous environment.arrow_forwardDraw structures corresponding to the following names:(a) 2,2,3-Trifluorobutanoic acid(b) 3-Hydroxybutanoic acid(c) 3,3-Dimethyl-4-phenylpentanoic acidarrow_forward
- draw 2 isomers tertiary amine with the formula C5H13Narrow_forwardDraw the structures of the following compounds:(a) Ethanoic acid(b) Bromopentane(c) Butanonearrow_forwardDraw structural formulas for the following the compounds: (a) Cis-1,3-diphenylcyclohexane (b) 5-phenylpentanoic acid (c) 3,4-dibromo-N,N-dimethylanilinearrow_forward
- The correct IUPAC name for CH2= CHCH2NHCH3is (i) Allylmethylamine (ii) 2-amino-4-pentene (iii) 4-aminopent-1-ene (iv) N-methylprop-2-en-1-aminearrow_forward(4) Write the structure of the following compounds from their IUPAC names. (a) ethanamide (b) methylethanoate (c) propanoic acid (d) 2-butanone (5) Write the structure of the following ethers and amines from their common names (a) methyl propyl (b) trimethylamine (6) Decide whether the following alcohols are polar or nonpolar (a) CH3CH2CH2CH2CH2CH2CH2OH (b) CH3CH2OHarrow_forwardDraw the structural formulas of the following compounds:(a) 2,3-Dimethylpentanal(b) 1,3-Dibromopropanone(c) 4-hydroxy-4-methylhexan-2-onearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





