
Concept explainers
(a)
Interpretation:
The given compound should be filled whether it is resonance structure, constitutional isomer, cis/trans isomer or same structure.
Concept introduction:
Isomer: Molecule has same molecular formula but different structural arrangement is called isomer.
Geometric isomerism (also known as E-Z isomerism or cis-trans isomerism): same molecular formula but different arrangement in the space. These isomers happen where you have restricted rotation in a molecule (double bond in the molecule). The
Constitutional isomer: (or structural) isomers differ in the connectivity they contain different functional groups and / or bonding patterns is called constitutional isomer.
Resonance: it is a process of delocalization electrons with in the molecule.
Chiral: Absence of a plane of symmetry or a center of symmetry is called chiral molecule, a non-superimposable on its mirror image is called chiral. A carbon atom is attached by the four different groups is called chiral carbon.
Enantiomers: Two stereoisomers that are mirror images of each other and they are non-supposable.
Diastereomers: Two stereoisomers that are non-mirror images of each other and they are non-supposable.
(a)
Answer to Problem 23.86QP
The given compound is diastereomers (a)
Explanation of Solution
To find: The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure
The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure needs to be known.
The given compound is diastereomers which is shown below.
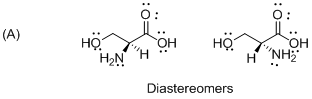
The above compounds are non-mirror images of each other and they are non-supposable therefore the given compound diastereomers.
(b)
Interpretation:
The given compound should be filled whether it is resonance structure, constitutional isomer, cis/trans isomer or same structure.
Concept introduction:
Isomer: Molecule has same molecular formula but different structural arrangement is called isomer.
Geometric isomerism (also known as E-Z isomerism or cis-trans isomerism): same molecular formula but different arrangement in the space. These isomers happen where you have restricted rotation in a molecule (double bond in the molecule). The functional groups are same side in the molecule is cis, the functional groups are opposite side is called trans isomer.
Constitutional isomer: (or structural) isomers differ in the connectivity they contain different functional groups and / or bonding patterns is called constitutional isomer.
Resonance: it is a process of delocalization electrons with in the molecule.
Chiral: Absence of a plane of symmetry or a center of symmetry is called chiral molecule, a non-superimposable on its mirror image is called chiral. A carbon atom is attached by the four different groups is called chiral carbon.
Enantiomers: Two stereoisomers that are mirror images of each other and they are non-supposable.
Diastereomers: Two stereoisomers that are non-mirror images of each other and they are non-supposable.
(b)
Answer to Problem 23.86QP
The given compound is diastereomers (b)
Explanation of Solution
To find: The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure
The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure needs to be known.
The given compound is diastereomers which is shown below.
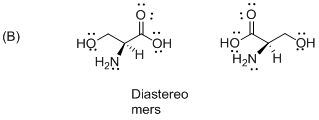
The above compounds are non-mirror images of each other and they are non-supposable therefore the given compound diastereomers.
(c)
Interpretation:
The given compound should be filled whether it is resonance structure, constitutional isomer, cis/trans isomer or same structure.
Concept introduction:
Isomer: Molecule has same molecular formula but different structural arrangement is called isomer.
Geometric isomerism (also known as E-Z isomerism or cis-trans isomerism): same molecular formula but different arrangement in the space. These isomers happen where you have restricted rotation in a molecule (double bond in the molecule). The functional groups are same side in the molecule is cis, the functional groups are opposite side is called trans isomer.
Constitutional isomer: (or structural) isomers differ in the connectivity they contain different functional groups and / or bonding patterns is called constitutional isomer.
Resonance: it is a process of delocalization electrons with in the molecule.
Chiral: Absence of a plane of symmetry or a center of symmetry is called chiral molecule, a non-superimposable on its mirror image is called chiral. A carbon atom is attached by the four different groups is called chiral carbon.
Enantiomers: Two stereoisomers that are mirror images of each other and they are non-supposable.
Diastereomers: Two stereoisomers that are non-mirror images of each other and they are non-supposable.
(c)
Answer to Problem 23.86QP
The given compound is resonance structure (c)
Explanation of Solution
To find: The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure
The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure needs to be known.
The given compound is resonance structure which is shown below.
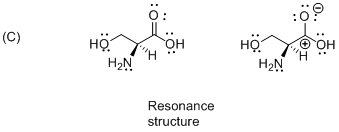
Isomers have different sequence of bond types or connection in different order is called as resonance structure therefore the given molecule is resonance structure.
(d)
Interpretation:
The given compound should be filled whether it is resonance structure, constitutional isomer, cis/trans isomer or same structure.
Concept introduction:
Isomer: Molecule has same molecular formula but different structural arrangement is called isomer.
Geometric isomerism (also known as E-Z isomerism or cis-trans isomerism): same molecular formula but different arrangement in the space. These isomers happen where you have restricted rotation in a molecule (double bond in the molecule). The functional groups are same side in the molecule is cis, the functional groups are opposite side is called trans isomer.
Constitutional isomer: (or structural) isomers differ in the connectivity they contain different functional groups and / or bonding patterns is called constitutional isomer.
Resonance: it is a process of delocalization electrons with in the molecule.
Chiral: Absence of a plane of symmetry or a center of symmetry is called chiral molecule, a non-superimposable on its mirror image is called chiral. A carbon atom is attached by the four different groups is called chiral carbon.
Enantiomers: Two stereoisomers that are mirror images of each other and they are non-supposable.
Diastereomers: Two stereoisomers that are non-mirror images of each other and they are non-supposable.
(d)
Answer to Problem 23.86QP
The given compound is constitutional isomer (d)
Explanation of Solution
To find: The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure
The given compound is resonance structure, constitutional isomer, cis/trans isomer or same structure needs to be known.
The given compound is constitutional isomer which is shown below.
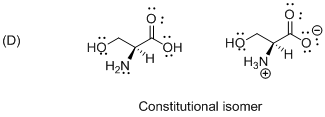
Isomers with the same order of connections and sequence of bond types, but which differ in the spatial arrangement of the atoms therefore the given molecule is called constitutional isomer
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Atoms First
- Why are different conformations of an alkane not considered structural isomers?arrow_forwardDistinguish between isomerism and resonance. Distinguish between structural and geometric isomerism. When writing the various structural isomers, the most difficult task is identifying which are different isomers and which are identical to a previously written structurethat is, which are compounds that differ only by the rotation of a carbon single bond. How do you distinguish between structural isomers and those that are identical? Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C4H8. Another common feature of alkenes and cycloalkanes is that both have restricted rotation about one or more bonds in the compound, so both can exhibit cis- trans isomerism. What is required for an alkene or cycloalkane to exhibit cis-trans isomerism? Explain the difference between cis and trans isomers. Alcohols and ethers are structural isomers of each other, as are aldehydes and ketones. Give an example of each to illustrate. Which functional group in Table 21-4 can be structural isomers of carboxylic acids? What is optical isomerism? What do you look for to determine whether an organic compound exhibits optical isomerism? 1-Bromo-1-chloroethane is optically active whereas 1-bromo-2-chloroethane is not optically active. Explain.arrow_forwardThere are 11 structures (ignoring stereoisomerism) with the formula C4H8O that have no carbon branches. Draw the structures and identify the functional groups in each.arrow_forward
- 2,2,4-trimethylpentane is the name of the 100 octane rating in gasoline. Select this molecule and any isomer(s) that contain a five-carbon chain and three methyl substituents (branches).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardWhat is cis-trans isomerism in alkenes? cis-trans isomerism in alkenes involves having the major groups in an alkene on the same or opposite side of the double bond cis-trans isomerism in alkenes involves having different or identical substituents at the both ends of the double bond cis-trans isomerism in alkenes involves having the major groups in an alkene in staggered or eclipsed conformations cis-trans isomerism in alkenes involves different location of double bond along the carbon chain How do the properties of cis-trans isomers differ from one another? cis and trans isomers have identical physical properites, whereas their chemical properties differ cis and trans isomers have identical chemical properites, whereas their physical properties differ cis and trans isomers have identical physical and chemical properties cis and trans isomers have distinct physical and chemical propertiesarrow_forward
- Indicate the relationship between the following pair of compounds as same, structural isomers, geometrical isomers, resonance or different searrow_forwardWhat is cis-trans isomerism in alkenes? cis-trans isomerism in alkenes involves having the major groups in an alkene on the same or opposite side of the double bond cis-trans isomerism in alkenes involves having different or identical substituents at the both ends of the double bond cis-trans isomerism in alkenes involves having the major groups in an alkene in staggered or eclipsed conformations cis-trans isomerism in alkenes involves different location of double bond along the carbon chainarrow_forwardWhich of the following compounds has a chiral carbon atom?arrow_forward
- What is the correct molecular structure for 1-cyclopropyl-3-ethyl-4-methyl hexane?arrow_forwardHow many structural isomers of C4H6 have exactly one ring?arrow_forwardIndicate for the following compound: (1) the number of carbons in the longest carbon chain, (2) the positions of the double bonds [separate # with comma], (3) the IUPAC name of the compound. Answer for blank # 1: 8 Answer for blank # 2: 2,5 Answer for blank # 3: 2,5-dimethyl The following compound exhibits cis/trans isomerism. Indicate for the compound: (1) the number of carbons in the longest chain, (2) the position of the double bond in the chain, (3) the IUPAC name of the compound [use the cis/trans in the name]. Answer for blank # 1: 6 Answer for blank # 2: 3 Answer for blank # 3: 3-hexenearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





