
Concept explainers
(a)
Interpretation:
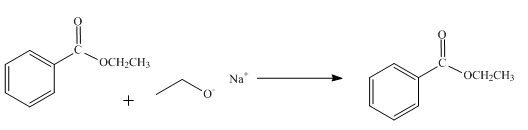
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
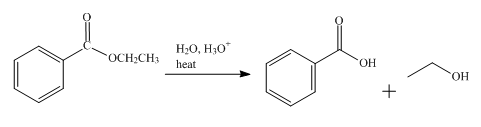
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reaction of ethyl benzoate in presence

Figure 1
The product obtained from the reaction of ethyl benzoate and
(b)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
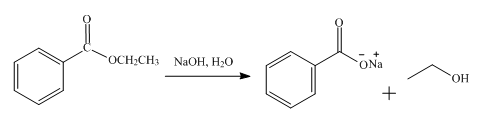
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reaction of ethyl benzoate in the presence of

Figure 2
The product obtained from the reaction of ethyl benzoate and
(c)
Interpretation:
The product obtained from the reaction of ethyl benzoate and aqueous
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
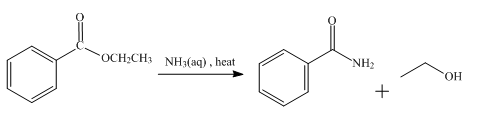
The product obtained from the reaction of ethyl benzoate and aqueous
Explanation of Solution
The reaction of ethyl benzoate in the presence of aqueous

Figure 3
The product obtained from the reaction of ethyl benzoate and aqueous
(d)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
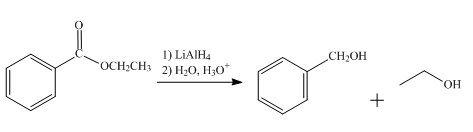
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reduction reaction of ethyl benzoate takes place in the presence of

Figure 4
The product obtained from the reaction of ethyl benzoate and
(e)
Interpretation:
The product obtained from the reaction of ethyl benzoate and excess
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of ethyl benzoate and excess
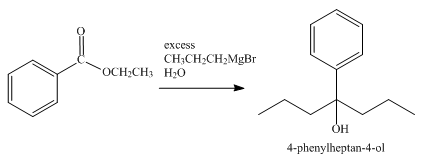
Explanation of Solution
The reaction of ethyl benzoate with an excess of Grignard reagent

Figure 5
The product obtained from the reaction of ethyl benzoate and excess
(f)
Interpretation:
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine at
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine, at
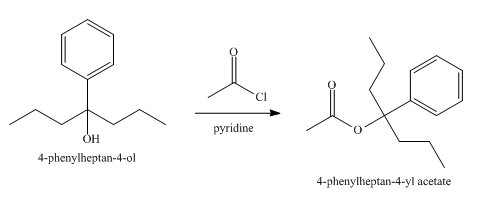
Explanation of Solution
The reaction of the product of part (e) and acetyl chloride, pyridine at

Figure 6
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine, at
(g)
Interpretation:
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is to be stated.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is shown below.
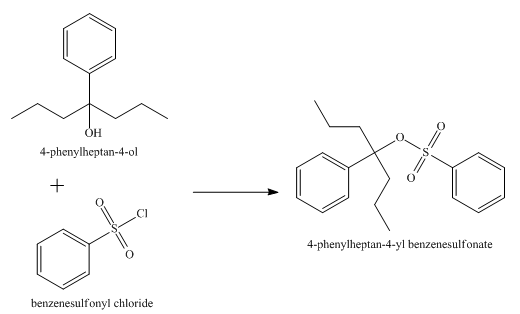
Explanation of Solution
The reaction of the product of part (e) and benzenesulphonyl chloride undergoes substitution reaction. It results in the formation of

Figure 7
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is shown in Figure 7.
(h)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reaction of ethyl benzoate with

Figure 8
The product obtained from the reaction of ethyl benzoate and
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- In a basic aqueous solution, esters react with hydroxide ion to form the salt of the carboxylic acid and the alcohol from which the ester is constituted. Name each of the following esters, and indicate the products of their reaction with aqueous base. (a) -OCH,CH3 (b) CH;CH2CH2-arrow_forwardDraw structural formulas for these ketones. (a) Ethyl isopropyl ketone (b) 2-Chlorocyclohexanone (c) 2,4-Dimethyl-3-pentanone (d) Diisopropyl ketone (e) Acetone (f) 2,5-Dimethylcyclohexanonearrow_forwardCompound X (C4H9Br) reacts by heating with NaOH in H2O to form Y. The compound Y then undergoes acid catalysed hydration by H2SO4 in 180°C to form 2-methyl prop-1-ene. (e) Determine the structure of X and Y. (f) Predict a MAJOR product when compound Y reacts with H2SO4 in 140°C. (g) Draw a structural isomer of X. Name the isomer using IUPAC nomenclature. (h) Describe a chemical test to distinguish between compound Y and 1-butanol.arrow_forward
- Write the products of the following acid-base reactions: (a) CH3OH + H2SO4 ² ? (b) CH3OH + NANH2 2 ? (c) CH3NH3+ Cl- + NaOH ?arrow_forward6) Which is the organic product for the following reaction? (a) (b) (c) (d) сон COOH ОН ОН COOH COOH KMnO4 H2Oarrow_forwardDraw the structure of the following compounds which parent names have been traced to a common name; (a)5-methyl-4-nitroimidazole (b)2-chloro-4-methoxythiazole.arrow_forward
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward(a) A hydrocarbon isolated from fish oil and from plankton was identified as 2,6,10,14-tetramethyl-2-pentadecene. Write its structure.(b) Alkyl isothiocyanates are compounds of the type RN C S. Write a structural formula for allyl isothiocyanate, a pungent-smelling compound isolated from mustard.(c) Grandisol is one component of the sex attractant of the boll weevil. Write a structural formula for grandisol given that R in the structure shown is an isopropenyl group.arrow_forwardThe odor of ripe bananas and many other fruits is due to the presence of esters. For example: Banana oil (isopentyl acetate) (a) Write the name (common or IUPAC) of the ester responsible for the fragrance of the following: pineapple, orange, apple, peach, & lavender (b) Choose one fragrant from (a) and name the alcohol and the carboxylic acid needed to synthesize this ester. (c) Show the detailed mechanism of the Fischer Esterification reaction that will be involved in the synthesis of the fragrant you have chosen in part (a).arrow_forward
- (a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forward(i) State reagents G and J. (ii) Draw the structural formula for compounds D, E and H.arrow_forwardDraw the reaction products of the following reactions of the compound 1-pentanol and name the compounds according to IUPAC! (a) B-elimination of H20 (b) SN reaction in the presence of hydrochloric acid (c) Condensation with acetic acid (d) Oxidation to the corresponding carbonyl compound (e) Condensation of two butanol moleculesarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
