
Interpretation:
The mechanism for the reaction of tert-butyl alcohol and 1-butanol under the reaction conditions in the experiment needs to be explained.
Concept Introduction:
Generally, alcohols do not react in simple nucleophilic displacement reactions. If alcohols are attracted directly by nucleophile, hydroxide ion which is a strong base can be displaced easily from the alcohol. But the reaction is not energetically favorable and cannot occur to an extent.
To avoid this, the nucleophilic substitution reaction of alcohol takes place in acidic medium. Initially, protonation of alcohol takes place followed by the removal of water molecule. The reaction is energetically stable.
This is represented as follows:
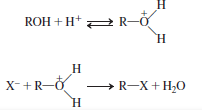
Explanation of Solution
This reaction examines the capability of two halides to replace an alcohol bynucleophilic substitution.
Initially, suppose the reaction of bromide and chloride ions with tert-butyl alcohol. Below possible reaction will take place:
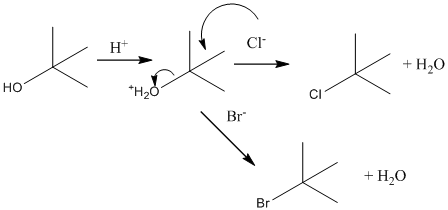
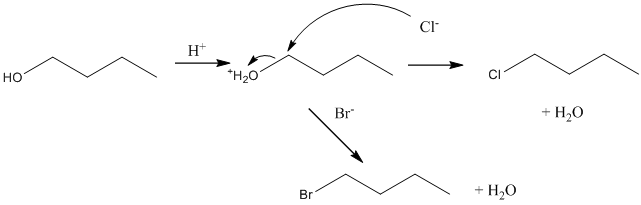
Now suppose, reaction of bromide and chloride ions with 1-butanol. Below possible reaction will take place:
Want to see more full solutions like this?
Chapter 20 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- What type of chemical intermediate plays an important role in causing primary, secondary, and tertiary alcohols to react at different rates in the Lucas test? Explain how this chemical intermediate controls the rates of reaction of the different alcohols.arrow_forward1) Based on the Material Safety Data Sheets (MSDS) for the compounds, which of the compounds in this experiment is the most hazardous, and why (you have been given a representative aryl halide rather than all of the alkenes – as the products are all new, there’s not a simple answer to gauge their potential hazard)? 2,3-dihydrofuran,2-Mercaptobenzimidazole,Selectfluor, Dimethylformamide,dichloromethane-methylene chloride.arrow_forwardImagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) In analyzing the products, you discover that the substitution pattern resulting from the reaction with phenol differs from the reaction with nitrobenzene. Explain this difference in substitution patterns using one or more judiciously selected reaction mechanisms or portions of mechanisms.arrow_forward
- Specify which compound from 2-methyl-2-butanol or 2-butanol and 1-Methylcyclopentanol or cyclohexanol compound pairs will convert to alkyl bromide faster with HBr. Write down the reactions for fast-converting alcoholsarrow_forwardConsidering the stoichiometry of the reaction (balance the equation!), what is the net oxidation state difference of the carbons in benzophenone going to benzopinacol? Remember that you have to calculate the oxidation state of the carbons in the product (benzopinacol) and subtract the oxidation state of the carbons in the reactants (benzophenones). The shortcut is to only calculate the oxidation states of the carbons that seem to have changed their bonding. Give a whole number answer with no units. hv OH Ph Ph OH Ph' Ph' Ph Ph OH Answer: -1arrow_forwardWrite a reaction that could be carried out to synthesize 1-(4-methylphenyl)ethanol (reactants will be a combination of Grignard reagent and carbonyl compound).arrow_forward
- Compare (and explain) the difference in reaction mechanism when primary alcohols are used instead of a tertiary alcohol.arrow_forwardComplete the electron‑pushing mechanism for the reaction of the γ‑hydroxyaldehyde in hydrochloric acid by adding any missing atoms, bonds, charges, nonbonding electrons, and curved arrows. Note the use of a generic alcohol representing another alcohol molecule in solution.arrow_forwardHow do you account for the observed solubility of naphthalene and sodium chloride in water and in ether based on the nature of the bonds that exist in them?arrow_forward
- Introduce Zaitseve's rule as it relates to the dehydration of 2-methylcyclohexanol and the major product of the reaction, 1 methylcyclohexanolarrow_forwardWrite the mechanism, as well as the structure of intermediate A and product B, for the following reaction.arrow_forwardDraw a flowchart showcasing the purification step done for the reaction under basic conditions (the basic reaction of 4-methoxyacetophenone with bleach, sodium hydroxide, and acetone), starting from the crude reaction mixture all the way to the isolated solid product. Assume for this drawing that the reaction was complete, and that none of the intermediates are found in the crude mixture. There may still be however traces of your starting material in the crude mixture, along with any excess reagentsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





