
(a)
Interpretation:
To write the molecular formula and condensed formula of given molecule:
Concept introduction:
Condensed formula: It is a way of writing the molecule where all the atoms are arranged in order as appear in structural formula but while writing condensed formula, bond lines are omitted. For example: Condensed formula of propane is CH3 CH2 CH3.
Molecular formula: It is the easiest way of representing the number of atoms of each element in a molecule.
While writing molecule formula,
Answer to Problem 48QAP
Condensed formula: CH3 COOH
Molecular formula: C2 H4 O2
Explanation of Solution
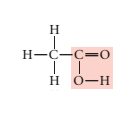
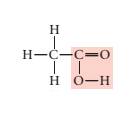
Given structural formula-
In above structural formula, CH3 group is attached with acetic acid group through carbon atom. Hence, its condensed formula will be CH3 COOH.
In 1 molecule of CH3 COOH, there are 2 carbon atoms, 4 hydrogen atoms and 2 oxygen atoms. Therefore, its molecular formula is C2 H4 O2.
(b)
Interpretation:
To write the molecular formula and condensed formula of given molecule:
Concept introduction:
Condensed formula: It is a way of writing the molecule where all the atoms are arranged in order as appear in structural formula but while writing condensed formula, bond lines are omitted. For example: Condensed formula of propane is CH3 CH2 CH3.
Molecular formula: It is the easiest way of representing the number of atoms of each element in a molecule.
While writing molecule formula, symbol of each element is written with their respective number of atoms in a single molecule. And these numbers are written in subscript as:
Answer to Problem 48QAP
Condensed formula: CH3 Cl
Molecular formula: CH3 Cl
Explanation of Solution
Given structural formula:
In above structural formula, CH3 group is attached with Chlorine group through carbon atom. Hence, its condensed formula will be CH3 Cl.
In 1 molecule of CH3 Cl, there are 1 carbon atom, 3 hydrogen atoms and 1 chlorine atom. Therefore, its molecular formula is CH3 Cl.
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry: Principles and Reactions
- Tell what is wrong with each of the following formulas and write a correct formula: a. HSH hydrogen sulfide b. HCLO2 chlorous acid c. 2HN2 hydrazine-two hydrogen atoms and four nitrogen atoms d. C2H6 ethanearrow_forwardContrast the two general types of chemical compounds in terms of their general physical properties.arrow_forwardTell what is wrong with each of the following molecular formulas and write a correct formula: a. H3PO3 phosphorous acid b. SICI4 silicon tetrachloride c. SOO sulfur dioxide d. 2HO hydrogen peroxide-two hydrogen atoms and two oxygen atomsarrow_forward
- What are the IUPAC names of the following compounds? manganese dioxide mercurous chloride ( Hg2Cl2) ferric nitrate [Fe( No 3)3] titanium tetrachioride cupric bromide (CuBr2)arrow_forwardWhat is the fundamental difference between an organic substance and an inorganic substance? Write chemical formulas of three inorganic molecules that contain carbon.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





