
Concept explainers
Interpretation:
The
Concept introduction:
An
Answer to Problem 2.11P
(A) The names of the functional groups from the given table that possess at least one
(B) The names of the functional groups from the given table which possess at least one
(C) The functional groups from the given table which possess no H-bond donors and no
Explanation of Solution
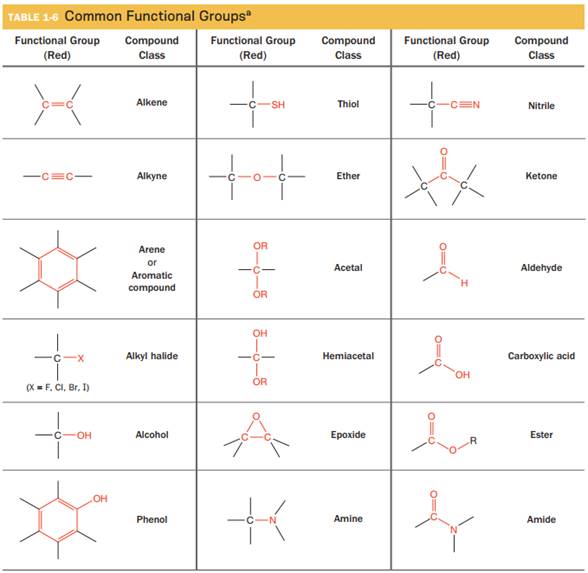
(A) The functional groups from the given table which possess at least one
- The given functional group is Nitrile:
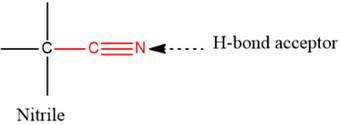
In this functional group, due to the high electronegativity of the N atom with a lone pair of electrons, there is one H-bond acceptor.
2) The given functional group is Ether:
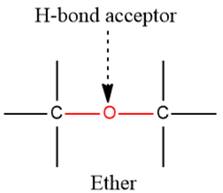
In this functional group, due to the high electronegativity of the O atom with a lone pair of electrons, there is one H-bond acceptor.
3) The given functional group is Ketone:
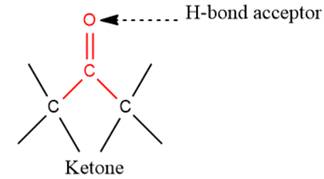
In this functional group, due to the high electronegativity of the O atom with a lone pair of electrons, there is one H-bond acceptor.
4) The given functional group is Acetal:
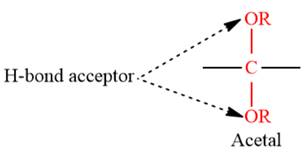
In this functional group, due to the high electronegativity of the O atom with a lone pair of electrons, there are two H-bond acceptors.
- The given functional group is Aldehyde:

In this functional group, due to the high electronegativity of the O atom with a lone pair of electrons, there is one H-bond acceptor.
- The given functional group is Alkyl halide (Only if X = F):
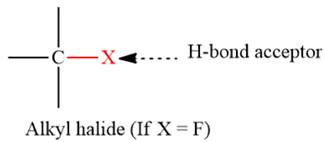
In this functional group, due to the high electronegativity of the F atom with a lone pair of electrons, there is one H-bond acceptor.
- The given functional group is Epoxide:
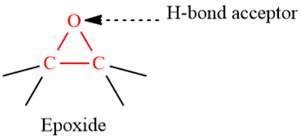
In this functional group, due to the high electronegativity of the O atom with a lone pair of electrons, there is one H-bond acceptor.
- The given functional group is Ester:
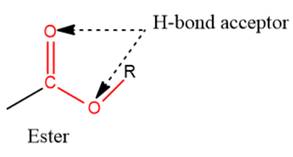
In this functional group, due to the high electronegativity of the O atoms with a lone pair of electrons, there are two H-bond acceptors.
- The given functional group is Amine:
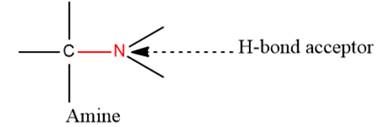
In this functional group, due to the high electronegativity of the N atom with a lone pair of electrons, there is one H-bond acceptor.
- The given functional group is Amide:
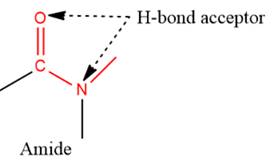
In this functional group, due to the high electronegativity of the N atom with a lone pair of electrons, there is one H-bond acceptor.
(B) The functional groups possessing at least one H-bond donor and one H-bond acceptor are:
1) The given functional group is Hemiacetal:
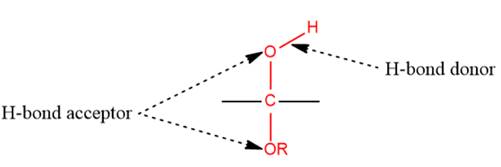
In this functional group, there is one H-bond donor and two H-bond acceptors. The high electronegativity of the O atom ensures that the O-H bond is highly polar and gives the H atom a large partial positive charge. This sets up a strong attraction between the H atom and the oppositely charged acceptor.
2) The given functional group is Carboxylic acid:
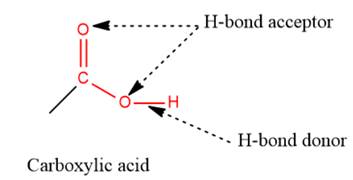
In this functional group, there is one H-bond donor and two H-bond acceptors. The high electronegativity of the O atom ensures that the O-H bond is highly polar and gives the H atom a large partial positive charge. This sets up a strong attraction between the H atom and the oppositely charged acceptor.
- The given functional group is Alcohol:
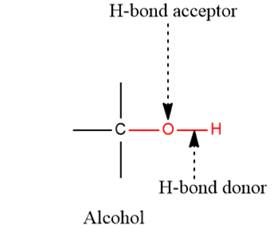
In this functional group, there is one H-bond donor and one H-bond acceptor. The high electronegativity of the O atom ensures that the O-H bond is highly polar and gives the H atom a large partial positive charge. This sets up a strong attraction between the H atom and the oppositely charged acceptor.
4) The given functional group is Phenol:
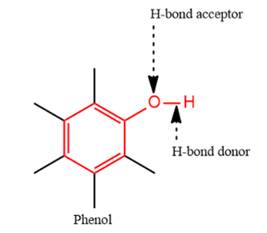
In this functional group, there is one H-bond donor and one H-bond acceptor. The high electronegativity of the O atom ensures that the O-H bond is highly polar and gives the H atom a large partial positive charge. This sets up a strong attraction between the H atom and the oppositely charged acceptor.
(C) The functional groups from the given table which possess no H-bond donor and no H-bond acceptor are:
1) The given functional group is Alkene:
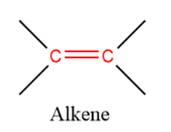
In this functional group, there is no H atom which is covalently bonded to a strongly electronegative atom such as N, O, or F. Therefore, there is no H-bond donor and H-bond acceptor.
2) The given functional group is Thiol:
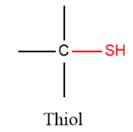
In this functional group, there is no H atom which is covalently bonded to a strongly electronegative atom such as N, O, or F. Therefore, there is no H-bond donor and H-bond acceptor.
3) The given functional group is Alkyne:

In this functional group, there is no H atom which is covalently bonded to a strongly electronegative atom such as N, O, or F. Therefore, there is no H-bond donor and H-bond acceptor.
- The given functional group is Arene or aromatic compound:
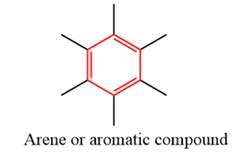
In this functional group, there is no H atom which is covalently bonded to a strongly electronegative atom such as N, O, or F. Therefore, there is no H-bond donor and H-bond acceptor.
- The given functional group is Alkyl halide (Only if X = Cl, Br, I):
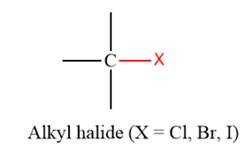
In this functional group, there is no H atom which is covalently bonded to a strongly electronegative atom such as N, O, or F. Therefore, there is no H-bond donor and H-bond acceptor.
The H-bond donor and H-bond acceptor of the given compounds are predicted on the basis of the number of electronegative atoms that are covalently bonded with hydrogen atoms and with a large concentration of negative charge and lone pair of electrons.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- answer 3 and 5. PLS MAKE IT LIKE THIS WAY SO THAT I UNDERSTAND IT IONIC YES OR NO POLAR YES OR NO NON POLAR YES OR NO IMF EXIST IS..... NO EXPLANATION NEEDED.arrow_forwardProblem (1) Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of those that do. CHF=CHF FC CH2 CH;=CH-CH,-CH3 -CHCH, -CHCHCH, CHCH,arrow_forwardProblem : IUPAC names? (a) CH3 (b) CH,CH,CH3 (c) CH3 CH3 (d) CH,CH3 (e) CH3 (f) Br CH(CH3)2 CH3 Br C(CH3)3 ©2004 Thomson - Brooks/Colearrow_forward
- points) The following molecule contains the aldehyde class of compounds. True or false? H True Falsearrow_forwardFor 1 and 2, draw the appropriate arrows to show the bond cleavage and bond formations in these reactions. Show the important nonbonding electrons.arrow_forwarddraw all the resonance structures for each of the above species. be sure to include the curved arrows that indicate which pair of electrons are shifted in going from one resonance structures to the next. draw resonance hybrid of each species.arrow_forward
- Which line (A or B) corresponds to the energy change for an ortho/para substitution?arrow_forwardEvery box should contain two structures. Be sure to include all lone pair electrons and nonzero formal charges. Step 1 Step 2 Step 3 Step 4 Draw H3O*, and then add the curved arrow notation showing an electrophilic addition of H*.arrow_forwardEach of the following compound is an amine ( ). Which line-angle formulas represent the same compound? Which represent constitutional isomers?arrow_forward
- How do I figure this out? Compound X is the reactant on the left.arrow_forwardFor 1 and 2, use curved arrows to illustrate the potential overall electron movements or bond changes, and identify the type of reaction by examining the overall chemical transformation. Show A-H bonds as needed.arrow_forwardProblem: Use the molecules below to answer the questions. (a) List one pair of enantiomers, if any. (b) List one pair of diastereomers, if any. (c) List one meso compound, if any. (d) How many total configurational isomers are possible for compound 2? онно CH 3 он но H QH H CH3 CH 3 CH3 H OH но. „CH3 H он H H. "CH 3 H H "CH3 H H3C H3C 4 3 1arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
