Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.4, Problem 18.2QC
Problem 19-2
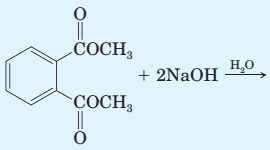
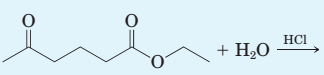
Complete the equation for each hydrolysis reaction. Draw all products as they are ionized under these experimental conditions.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
urgent please help i am learning Carboxylic Acid Derivative Reactions. what is the product of the bellow. pls crcle the answer
Problem 15 of 85
Submit
Draw the major product of this reaction.
Ignore inorganic byproducts.
H+, Br2 (1 equiv)
Select to Draw
>
Question 7 bint)
a) Listen
Both acetic acid and phenol will react with aqueous NaOH to yield a salt, but only
acetic acid will react with aqueous sodium bicarbonate, NaHCO3. This is because
sodium hydroxide is too weak a base to react with both compounds.
acetic acid is a weaker acid than phenol, and so reacts well with sodium
bicarbonate, the weaker base.
phenol is a weaker acid than acetic acid.
sodium bicarbonate does not react well with compounds that have benzene
rings.
Question 74
E) Listen
What is the best description of the functional group in the following structure?
CH,
он
a hemiacetal
a secondary alcohol
an ether
an acetal
Question 75 (1
E) Listen
What is the IUPAC name of the following compound?
CH,
CH-C
CH3
sodium isopropanoate
sodium 2-methylpropanoate
2-methyl-1-sodium propanoate
O 2-methyl-3-sodium propanoate
Chapter 18 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 18.1 - Prob. 18.1QCCh. 18.4 - Problem 19-2 Complete the equation for each...Ch. 18.4 - Prob. 18.3QCCh. 18 - Prob. 1PCh. 18 - Write the IUPAC name for each compound.Ch. 18 - Prob. 3PCh. 18 - Prob. 4PCh. 18 - Prob. 5PCh. 18 - Prob. 6PCh. 18 - 0 Complete the equations for these reactions.
Ch. 18 - Prob. 8PCh. 18 - Prob. 9PCh. 18 - Prob. 10PCh. 18 - Prob. 11PCh. 18 - Prob. 12PCh. 18 - 6 Why are Dacron and Mylar referred to as...Ch. 18 - 7 What type of structural feature do the...Ch. 18 - Prob. 15PCh. 18 - Prob. 16PCh. 18 - 0 Show how triphosphoric acid can form from three...Ch. 18 - 1 Write an equation for the hydrolysis of...Ch. 18 - Prob. 19PCh. 18 - (The Pyrethrins-Natural Insecticides of Plant...Ch. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - Prob. 24PCh. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - Prob. 34PCh. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - Prob. 40PCh. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Prob. 44PCh. 18 - Prob. 45PCh. 18 - Prob. 46P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the products obtained by the hydrolysis of the given compound. Ο Ο || || -COC O o one molecule of dibenzoic acid and one molecule of benzene two molecules of benzoic acid O one molecule of benzene and one molecule of a carboxylic acid two molecules of phenol Previous Page Next Page Page 29 aarrow_forwardProblem 16 of 32 Draw the two products of the reaction shown below. Ignore inorganic byproducts. H3C Done conc. HCIarrow_forwardWhat is the best reason why carboxylic acid 3 is the most acidic? aioH cioH а он дон CI- OH OH 1 || F ||| IV A) It is the least sterically hindered. B) The F groups are good leaving groups. C) F is very electronegative and can stabilize a negative charge as FO. D) F is very electronegative and will help to stabilize a negative charge on oxygen via induction. E) The conjugate base is stabilized by resonance.arrow_forward
- Is A, C, D or E acid base or no?arrow_forwardPro Question: Propose a synthesis for this amine by the Gabriel reaction. Show all starting materials, reagents, conditions, and structures of by-products. NH₂arrow_forwardProblem 18 of 28 Draw the product of the reaction shown below at physiological pH (pH = 7.4). Ignore inorganic byproducts. N. IZ H CF3CO2H Submit Qarrow_forward
- Review: Which of the following contribute to the favorablilty of the following elimination? H Ph yo H H CH3OH + • Ph + CI Hint: the "favorability" of a reaction has nothing to do with the speed of a reaction. When I ask about favorability, I am asking about thermodynamic considerations rather than kinetic ones. The unstable charge on the base lowers the activation energy of the reaction. Formation of three products from two reactants is entropically favorable The charge formed on the leaving group is more stable than the charge on the base. The pi bond formed in the product is more stable than the sigma bond broken in the reactantarrow_forwardProblem 22 of 44 Draw the major product of this reaction. Ignore inorganic byproducts. Submit Assume that the water side product is continuously removed to drive the reaction toward products. PhNHNH2, TSOH Select to Drawarrow_forwardProblem 4. Each of the following reactions is displaced to the right. Make a list of all the Bronsted acids that appear in these equations with these acids arranged according to decreasing acid strength. Make a similar list for Bronsted bases. (a) HCO3 + OH H2O + CO32- (b) HC2H3O2 + HS = H2S + C2H3O2" (c) H2S + CO32- = HCO3 + HS- (d) HSO4 + C2H3O2 HC2H3O2 + SO42-arrow_forward
- ar Rank the following compounds in terms of increasing acidity of the most acidic proton. Assume all conjugated atoms are coplanar. Check A D X S B Q Search C Save For Later © 2023 McGraw Hill LLC. All Rights Reserved Terms of Use | Parrow_forwardQUESTION 20 What is the conjugate acid of CH3NH2? CH3N+ CH3N− CH3NH2 does not have a conjugate acid. CH3NH+ CH3NH3 CH3NH3− CH3NH− CH3NH3+arrow_forwardProblem 15 of 25 Draw the product of this reaction. Ignore inorganic byproducts. Br2 (2 equiv) Select to Drawarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY