
(a)
Interpretation:
The complete, detailed mechanism of a given reaction in mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to
Answer to Problem 18.57P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
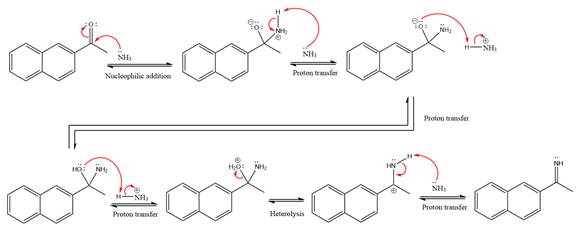
Explanation of Solution
The given reaction is
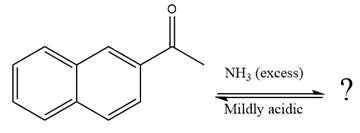
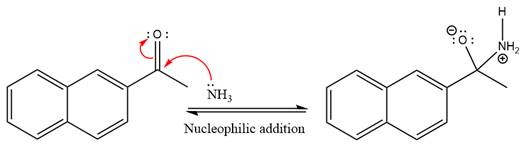
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and the excess ammonia acts as the nucleophile. Ammonia attacks the
First three steps are the nucleophilic addition reactions on the ketone. In the first step, nucleophile
In the next step, deprotonation takes place by ammonia
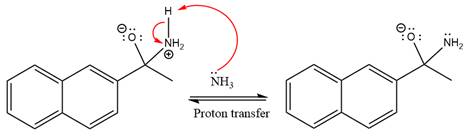
Next, negativively charged oxygen ion abstracts the proton from the ammonium ion.
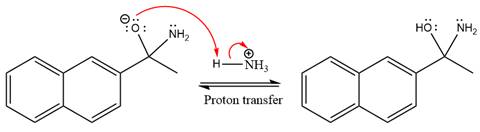
The lone pairs on
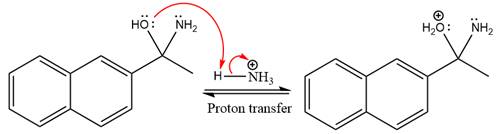
The
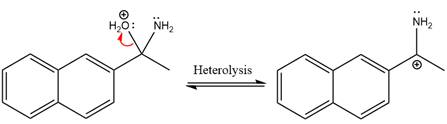
Ammonia nucleophile abstracts the proton from the amino group and forms imine as the product.
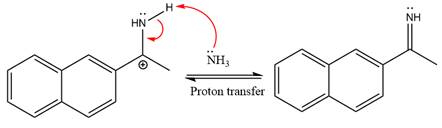
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
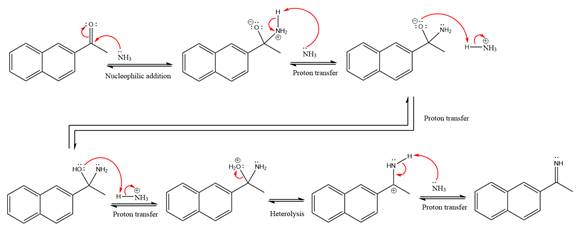
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess ammonia is drawn.
(b)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
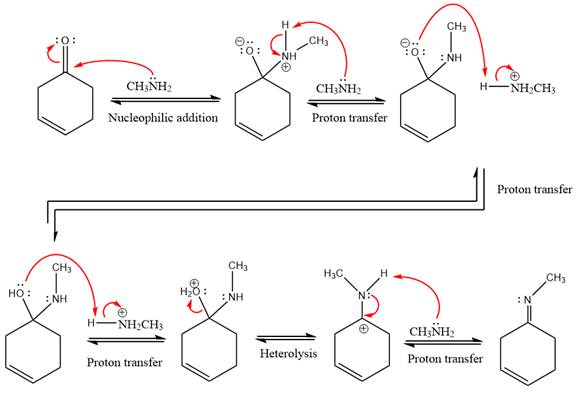
Explanation of Solution
The given reaction is
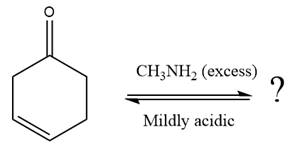
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and excess methylamine acts as the nucleophile.
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
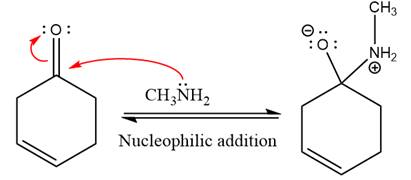
In the next step, deprotonation takes place by the ammonia
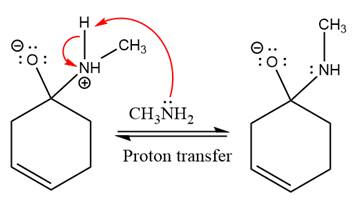
Next, negativively charged oxygen ion abstracts the proton from methylamino ion.
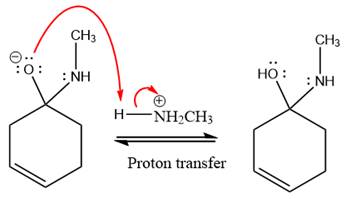
The lone pairs on
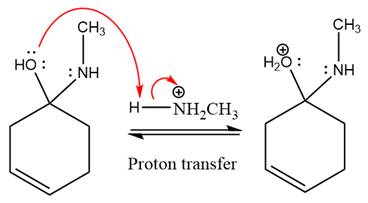
The
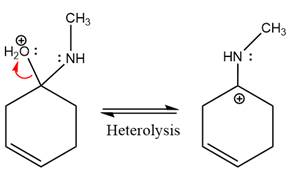
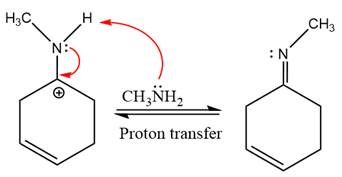
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
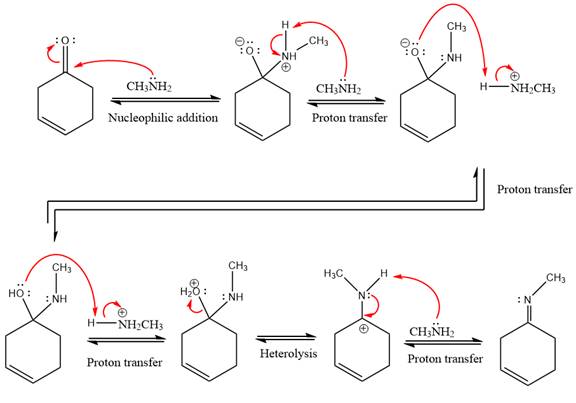
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess methylamine is drawn.
(c)
Interpretation:
The complete, detailed mechanism of a given reaction under acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an ketone is the major product.
Explanation of Solution
The given reaction is
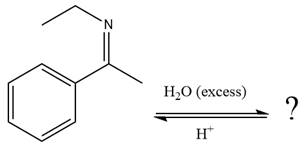
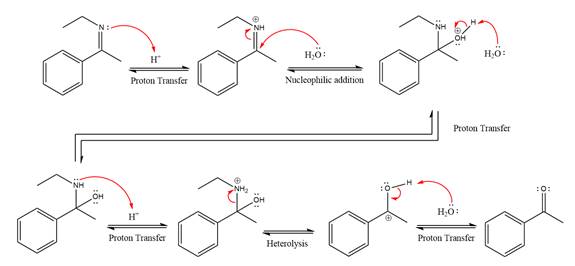
This is an imine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step, the
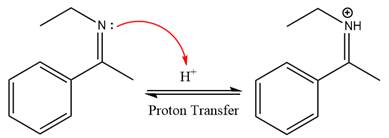
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
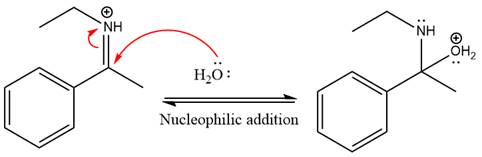
In the next step, deprotonation takes place by the nucleophile produced
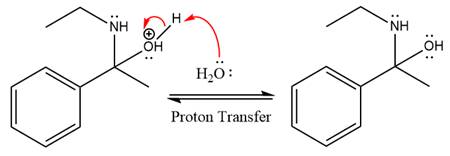
The lone pairs on
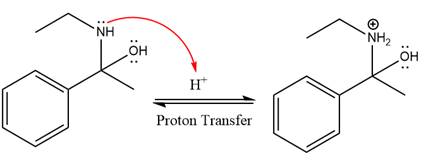
The
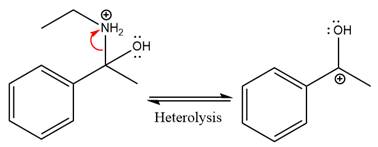
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
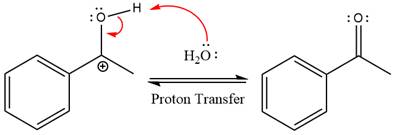
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
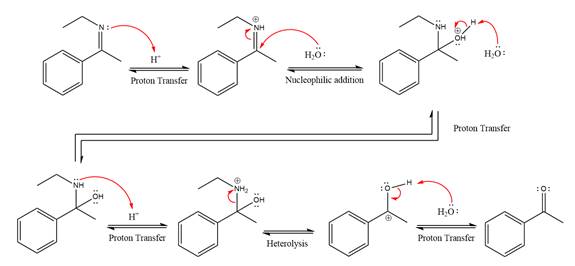
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
(d)
Interpretation:
The complete, detailed mechanism of the given reaction in the mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.
Explanation of Solution
The given reaction is
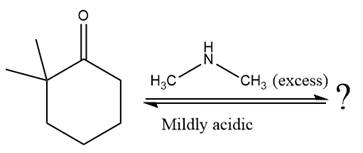
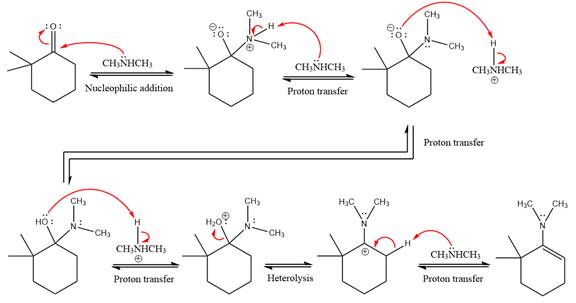
This is an enamine formation reaction in which the reaction is catalyzed under mildly acidic conditions, and the secondary
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
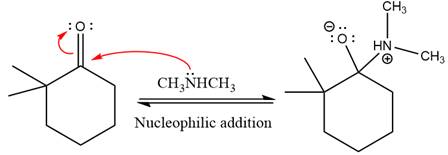
In the next step, deprotonation takes place by
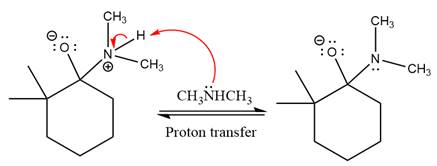
Next, negatively charged oxygen ion abstracts the proton from dimethylamino ion.
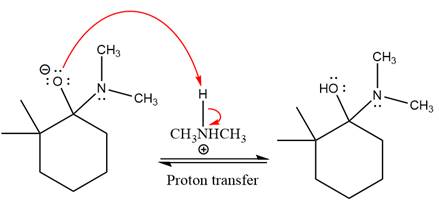
The lone pairs on
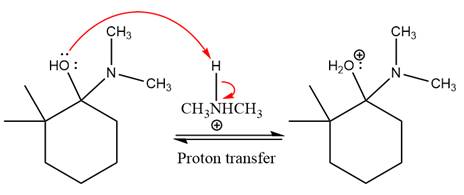
The
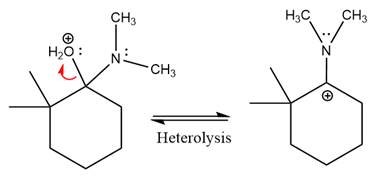
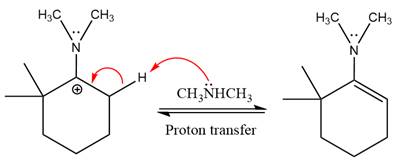
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.

The complete, detailed mechanism of the given reaction under acidic medium and excess alcohol is drawn.
(e)
Interpretation:
The complete, detailed mechanism of a given reaction under strongly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
Explanation of Solution
The given reaction is
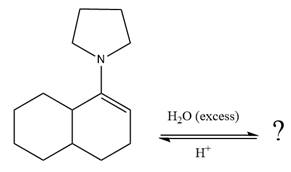
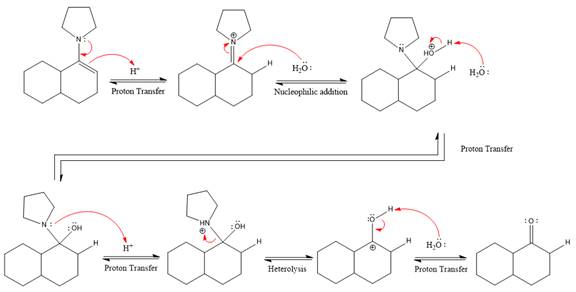
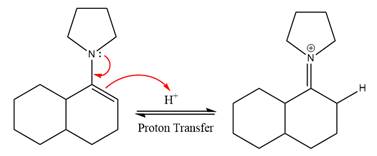
This is an enamine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step,
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
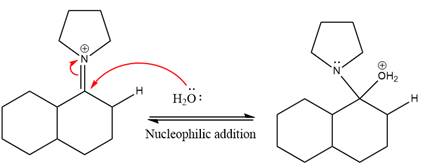
In the next step, deprotonation takes place by the nucleophile produced
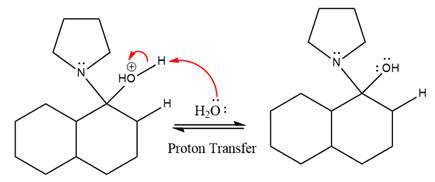
The lone pairs on
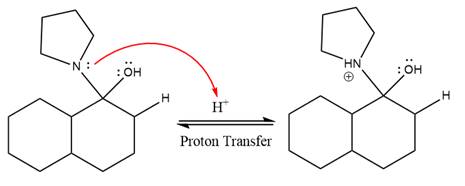
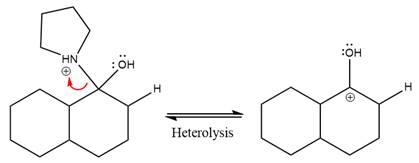
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
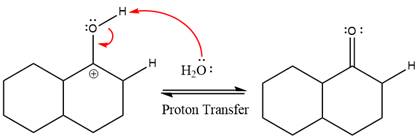
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
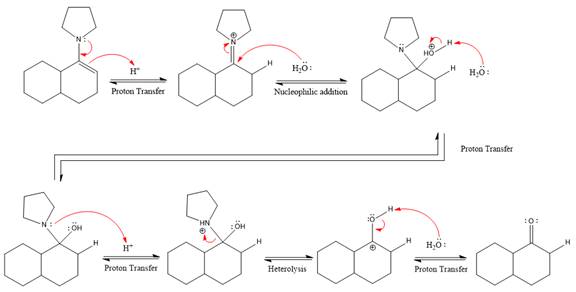
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





