
Concept explainers
Interpretation:
The infrared spectra for carvone and limonene needs to be interpreted. Also, the proton and carbon-13 NMR spectra of carvone needs to be explained.
Concept Introduction:
In the IR spectroscopy, the IR region of the
Nuclear Magnetic Resonance (NMR) spectroscopy is a logical chemistry method used in quality control.
It studies for defining the material and purity of a sample and their molecular structure. It explains magnetic energy levels undergoing the resonance transition when the atomic nuclei are exposed to an external magnetic field and an electromagnetic radiation is applied with the specific frequency. The NMR spectrum is obtained by detecting the absorption signals.
Explanation of Solution
The structure of limonene is represented as follows:
Limonene will have C-H stretching at 3000 cm-1 and C=C stretching at 1645 cm-1
Now, the structure of Carvone is represented as follows:
Carvone will have C=C stretching at 1645 cm-1 and a, β-unsaturated
The structures of Carvone are given below along with the NMR interpretation (both 1H and 13C).
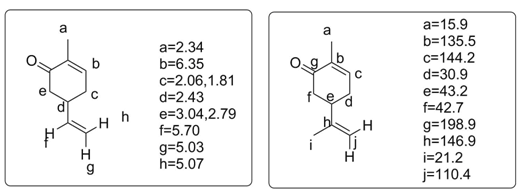
Carvone NMR (1H and 13C)
In the NMR of Carvone two methyl peaks are there along with vinylic protons. There are few CH2 protons. In 13-C there are a, β-unsaturated ketone peak along with C=C peaks. Two methyl peaks and CH2 peaks.
In NMR of limonene there are two methyl peaks, few CH2 peaks and vinylic protons. In 13-C there are two methyl along with C=C and CH2 peaks.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Ketones undergo a reduction when treated with sodium borohydride, NaBH4. What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm-1 and M+=74 in the mass spectrum?arrow_forwardIllustrate how diazomethane and ozone can both behave as 1,3-dipoles. Indicate, by drawing their resonance forms, how they can do so and draw the products of their reaction with styrenearrow_forwardSpecialized reagents and their acronyms: MCPBA= meta-chloroperbenzoic acid; HOAC = acetic acid, NaOAc = Sodium Acetate; Ph= phenyl = C6H5-; P(Ph)3 = triphenyl phosphine; NBS = N- bromosuccinimide, PBR3, SOCI2, AlCl3, FeBr3, H2SO4, Li, Na, Mg, Br2, CrO3, LDA = lithium diisopropylamide, PCC = pyridinium chlorochromate, KO-C(CH3)3, LAH = LIAIH4, (sia)2BH = disecondary isobutyl borane, KMNO4, HIO4 = periodic acid %3D %3D %3D %3D %3D write the missing reagents and solvents over the arrows. Some transformation require multiple steps. In some cases there will be multiple arrows. Br Brarrow_forward
- 5A Explain which of the following statements are true and which are false. i. N,N'-dicyclohexylurea (DCU) is the dehydrating reagent that converts a carboxylic acid to the corresponding anhydride. ii. The fact that carbonyl compounds have a lower boiling point than alcohols with the same C atoms is because the molecular dipole moment of carbonyl compounds is less than that of alcohols. iii. 3-Methylbut-2-en-2-ol is the only tautomeric structure of 3- methylbutan-2-one.arrow_forwardSpecialized reagents and their acronyms: MCPBA= meta-chloroperbenzoic acid; HOAC = acetic acid, NaOAc = Sodium Acetate; Ph= phenyl = C6H5-; P(Ph)3 = triphenyl phosphine; NBS = N- bromosuccinimide, PBR3, SOCI2, AICI3, FeBr3, H2SO4, Li, Na, Mg, Br2, CrO3, LDA = lithium diisopropylamide, PCC = pyridinium chlorochromate, KO-C(CH3)3, LAH = LIAIH4, (sia)2BH = disecondary isobutyl borane, KMNO4, HIO4 = periodic acid %3D write the missing reagents and solvents over the arrows. Some transformation require multiple steps. In some cases there will be multiple arrows. Ph -Ph Ph Ph Brarrow_forwardSpecialized reagents and their acronyms: MCPBA= meta-chloroperbenzoic acid; HOAC = acetic acid, NaOAc = Sodium Acetate; Ph= phenyl = C6H5-; P(Ph)3 = triphenyl phosphine; NBS = N- bromosuccinimide, PBR3, SOCI2, AICI3, FeBr3, H2SO4, Li, Na, Mg, Br2, CrO3, LDA = lithium diisopropylamide, PCC = pyridinium chlorochromate, KO-C(CH3)3, LAH = LIAIH4, (sia)2BH = disecondary isobutyl borane, KMNO4, HIO4 = periodic acid %3D %3D %3D %3D %3D %3D %3D write the missing reagents and solvents over the arrows. Some transformation require multiple steps. In some cases there will be multiple arrows. ОН H. (R) Br (S) HO OHarrow_forward
- Although benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of substituted benzenes absorb either upfield or downfield from this value depending on the substituent. Explain the observed values for the carbon ortho to the given substituent in the monosubstituted benzene derivatives X and Y.arrow_forwardPropose a method to separate a mixture containing phenol, benzoic acid, naphthalene, and p-nitroaniline. Phenol is soluble in sodium hydroxide solution but insoluble in neutral water or sodium bicarbonate solution. Benzoic acid is soluble in either sodium hydroxide or sodium bicarbonate solutions. Write out the structures of the molecules in your scheme.arrow_forwardSpecialized reagents and their acronyms: MCPBA= meta-chloroperbenzoic acid; HOAC = acetic acid, NaOAc = Sodium Acetate; Ph= phenyl = C6H5-; P(Ph)3 = triphenyl phosphine; NBS = N- bromosuccinimide, PBr3, SOCI2, AICI3, FeBr3, H2SO4, Li, Na, Mg, Br2, CrO3, LDA = lithium diisopropylamide, PCC = pyridinium chlorochromate, KO-C(CH3)3, LAH = LIAIH4, (sia)2BH = disecondary isobutyl borane, KMNO4, HIO4 = periodic acid %3D %3D %3D %3D %3D write the missing reagents and solvents over the arrows. Some transformation require multiple steps. In some cases there will be multiple arrows. Ph CH CH o 0om ilubongo CEC-Ph In questions 14-24, write the products of the following transformations. If there is no reaction write NR. Indicate R or S or racemic or indicate cis or trans where appropriate OH + LDA THF solvent (S) Pharrow_forward
- Following are 1H-NMR spectra for compounds B (C6H12O2) and C (C6H10O). Upon warming in dilute acid, compound B is converted to compound C. Deduce the structural formulas for compounds B and C.arrow_forwardIdentify the structures of D and E, isomers of molecular formula C6H12O2, from their IR and 1H NMR data. Signals at 1.35 and 1.60 ppm in the 1H NMR spectrum of D and 1.90 ppm in the 1H NMR spectrum of Eare multiplets.a. IR absorption for D at 1743 cm−1b. IR absorption for E at 1746 cm−1arrow_forwardTreatment of compound C (molecular formula C9H12O) with PCC affords D (molecular formula C9H10O). Use the 1H NMR and IR spectra of D to determine the structures of both C and D.arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



