
Concept explainers
(a)
Interpretation: Starting material and reagent used to prepare the given thiols need to be identified.
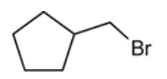
Concept introduction: preparation of thiol can be done when
(b)
Interpretation: Starting material and reagent used to prepare the given thiols need to be identified.
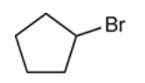
Concept introduction: preparation of thiol can be done when alkyl halide reacts with sodium hydrosulfide. The reaction follows the SN2 mechanism. Here, the substitution of the SH group takes place, and halide act as leaving group. Since it is an SN2 reaction, thus, inversion of the configuration takes place.
(c)
Interpretation: Starting material and reagent used to prepare the given thiols need to be identified.
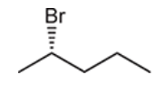
Concept introduction: preparation of thiol can be done when alkyl halide reacts with sodium hydrosulfide. The reaction follows the SN2 mechanism. Here, the substitution of the SH group takes place, and halide act as leaving group. Since it is an SN2 reaction, thus, inversion of the configuration takes place.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardWhat is the IUPAC name of the following compound? 4-phenyl-3-hexanone O 3-phenyl-4-hexanone O 1,2-diethyl-2-phenylethanal O 1-methyl-1-phenyl-2-butanonearrow_forward
- Give the name of a carboxylic acid or carboxylate salt used in each of the following ways: a.As a soap b.As a general food preservative used to pickle vegetables c.As a preservative used in soft drinks d.As a treatment of athletes foot e.As a mold inhibitor used in bread. f.As a food additive noted for its pH buffering abilityarrow_forward18-18 Propanoic acid and methyl acetate are constitutional isomers, and both are liquids at room temperature. One of these compounds has a boiling point of 141°C; the other has a boiling point of 57°C. Which compound has which boiling point? Explain.arrow_forwardWhat two functional groups react to form the following? a. A hemiacetal b. An acetal c. A ketal d. A hemiketalarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





