
(a)
Interpretation:
The alcohol should be identified when the given 2-methylpropanal undergoes reduction with sodium borohydrate.
Concept introduction:
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
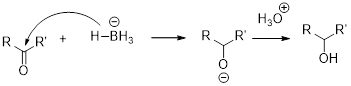
(b)
Interpretation:
The alcohol should be identified when the given cyclohexanone undergoes reduction with sodium borohydrate.
Concept introduction:
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
Aldehydes and ketons react with sodium borohydrate undergoes reduction to forms alcohols.
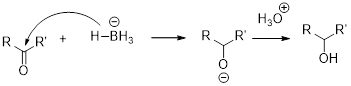
(c)
Interpretation:
The alcohol should be identified when the given tert-butylcyclohexanone undergoes reduction with sodium borohydrate.
Concept introduction:
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
Aldehydes and ketons react with sodium borohydrate undergoes reduction to forms alcohols.
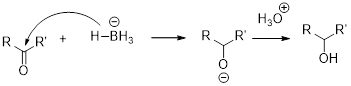
(d)
Interpretation:
The alcohol should be identified when the given methyl phenyl
Concept introduction:
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
Aldehydes and ketons react with sodium borohydrate undergoes reduction to forms alcohols.
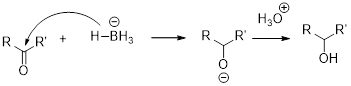
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Essential Organic Chemistry (3rd Edition)
- When phenol is treated with excess of bromine water, which of the following product it gives? Select one: a. 2-Bromophenol b. 2,4-Dibromophenol c. 2,4,6-Tribromophenol d. 2,3,4-Tribromophenolarrow_forwardReactions of carbonyl compounds with hydride ion donors. a. Draw reaction of an aldehyde with sodium borohydride forms a primary alcohol. b. Draw reaction of a ketone with sodium borohydride forms a secondary alcohol.arrow_forwardStarting with cyclohexanone, show how to prepare these compounds. In addition to the given starting material, use any other organic or inorganic reagents as necessary. a. Cyclohexanol b. Cyclohexene c. cis-1,2-Cyclohexanediol d. 1-Methylcyclohexanol e. 1-Methylcyclohexene f. 1-Phenylcyclohexanol g. 1-Phenylcyclohexene h. Cyclohexene oxide i. trans-1,2-Cyclohexanediolarrow_forward
- The reaction of an alkyl halide with aceto acetic ester results to the production of alan O A. ketone O B. secondary alcohol C. primary alcohol O D. acidarrow_forwardGive the structure corresponding to each name. a. 7,7-dimethyl-4-octanol b. 5-methyl-4-propyl-3-heptanol c. 2-tert-butyl-3-methylcyclohexanol d. trans-1,2-cyclohexanediolarrow_forwardWhich of the following forms a 1° alcohol when reacted with Grignard reagent? A. ketone B. carbon dioxide C. formaldehyde D. acyl halidearrow_forward
- Aldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forwardPhosgene (COCl2) was used as a poison gas in World War I. What product would be formed from the reaction of phosgene with each of the followingreagents?a. one equivalent of methanol b. excess methanol c. excess propylamine d. excess waterarrow_forwardWhich of the following is the most correct name for this compound? H a. 2,5-dioxooctanal b. 4,7-dioxooctanedione c. 3,6-dioxooctanal d. 3,6-dioxooctanedione e. 4,7-dioxooctanalarrow_forward
- 10. What is the IUPAC name for the compound shown below? CH3 CH,--CH-CH-CH3 A. 2-methyl-4-pentanone C. 2-methyl-pantanol B. 4-methyl-2-pentanone D. methyl-2-methyl-propanoic acidarrow_forwardWhat is the IUPAC name of the following compound? Br CH3 H3C O A. 5-Bromo-2-methylphenyl ethanoate B. 3-Bromo-6-methylphenyl ethanoate C. 4-Bromo-2-{oxy-(1-oxoethyl)} toluene EO D. Methyl-5-bromo-2-methyl benzoatearrow_forwardCompare aldehydes and ketones as to (Use acetaldehyde and acetone as examples). 1. Reaction with Tollen’s reagent 2. Reaction with Fehling’s reagent 3. Reaction with dilute NaOHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
