
(a)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 37E
The electron dot formula for
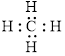
The structural formula for
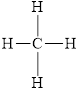
Explanation of Solution
The total number of valence electron in
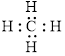
Figure 1
All electron pair are involved in bonding. Therefore, there is no lone pair. The structural formula for
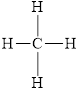
Figure 2
The electron dot formula for
The structural formula for
(b)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 37E
The electron dot formula for
![]()
The structural formula for
![]()
Explanation of Solution
The total number of valence electron in
![]()
Figure 3
Out of ten electron pairs, only two electron pairs are involved in bonding. Therefore, there are eight lone pairs. The structural formula for
![]()
Figure 4
The electron dot formula for
The structural formula for
(c)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 37E
The electron dot formula for
![]()
The structural formula for
![]()
Explanation of Solution
The total number of valence electron in
![]()
Figure 5
Out of seven electron pairs, three electron pairs are involved in bonding. Therefore, there are four lone pairs. The structural formula for
![]()
Figure 6
The electron dot formula for
The structural formula for
(d)
Interpretation:
The electron dot formula and structural formula for
Concept introduction:
The electron dot formula shows the valence electrons which form the bond between the atoms in a molecule. The electron pairs that are shared by the atoms are known as bonding electrons. The other electrons that are present in order to complete the octet are known as non-bonding electrons. The electron dot formula is also known as the Lewis structure.
Answer to Problem 37E
The electron dot formula for
![]()
The structural formula for
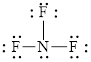
Explanation of Solution
The total number of valence electron in
![]()
Figure 7
Out of thirteen electron pairs, only three electron pairs are involved in bonding. Therefore, there are ten lone pairs. The structural formula for
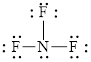
Figure 8
The electron dot formula for
The structural formula for
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Draw a Lewis structure for each of the following molecules and ions. In each case, the atoms can be connected in only one way. (a) Br2 (b) H2S (c) N2H4 (d) N2H2 (e) CN- (f) NH4+ (g) N2 (h) O2arrow_forward1) Using principles of chemical bonding and/or intermolecular forces, explain each of the following. (a) Xenon has a higher boiling point than neon has. (b) Solid copper is an excellent conductor of electricity, but solid copper chloride is not. (c) SiO2 melts at a very high temperature, while CO2 is a gas at room temperature, even though Si and C are in the same chemical family. (d) Molecules of NF3 are polar, but those of BF3 are not. 2) Using principles of chemical bonding and/or intermolecular forces, explain each of the following: (a) MgCl2 and SiCl4 (b) MgCl2 and MgF2 (c) F2 and Br2 (d) F2 and N2 Note: Please Briefly Explainarrow_forwardWrite the chemical formula for the ionic compound formed from each pair of ions.(a) Mg2+ and I-(b) Na+ and O2-arrow_forward
- Which statements are true about electronegativity? (a) Electronegativity increases from left to right in a period of the Periodic Table. (b) Electronegativity increases from top to bottom in a column of the Periodic Table . (c) Hydrogen, the element with the lowest atomic number, has the smallest electronegativity. (d) The higher the atomic number of an element, the greater its electronegativity.arrow_forwardAnswer the following questions that relate to the chemistry of nitrogen. (a) Two nitrogen atoms combine to form a nitrogen molecule, as represented by the following equation. 2 N(g) ® N2(g) Using the table of average bond energies below, determine the enthalpy change, AH, for the reaction. Average Bond Energy (k) mol-1) Bond N-N 160 N=N 420 N°N 950 (b) The reaction between nitrogen and hydrogen to form ammonia is represented below. N2(g) + 3 H2(g)® 2 NH3(g) AH° = -92.2 kJ Predict the sign of the standard entropy change, AS', for the reaction. Justify your answer. (C) The value of AG° for the reaction represented in part (b) is negative at low temperatures but positive at high temperatures. Explain.arrow_forwardWrite the chemical formulas for the following compounds:(a) Silver cyanide(b) Calcium hypochlorite(c) Potassium chromate(d) Gallium oxide(e) Potassium superoxide(f) Barium hydrogen carbonatearrow_forward
- Predict which of these compounds are ionic and which are covalent.(A) Ca3N2(B) Li2CO3(C) PCl5(D) NaOH(E) CH4(F) MgOarrow_forwardExplain the chemical bonding between Nitrogen(1N) and Bromine (3Br) using Lewis electron dot structure. Answer the following for the structure given below. HẠC "CH3 b() no of bond pair electrons b(i) no of lone pair electrons b(ii) no of single bond b(iv) no of double bondarrow_forwardName each ionic compound. In each of these compounds,the metal forms only one type of ion. (a) CsCl (b) SrBr2 (c) K2O (d) LiFarrow_forward
- Write an electron configuration for each element and the corresponding Lewis structure. Indicate which electrons in the electron configuration are included in the Lewis structure.(a) N(b) C(c) Cl(d) Ararrow_forwardWhich of the following statements are true regarding an ionic bond between calcium and oxygen in an CaO formula unit? (a) Calcium ions and oxide ions bond by covalent attraction. (b) Calcium atoms lose electrons and oxygen atoms gain electrons. (c) The ionic radius of a calcium ion is less than its atomic radius. (d) Breaking an ionic bond between calcium and oxygen releases energy.arrow_forwardWrite a formula for each of the following compounds:(a) silicon dioxide(b) silicon tetraiodide(c) silane(d) silicon carbide(e) magnesium silicidearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





