
Concept explainers
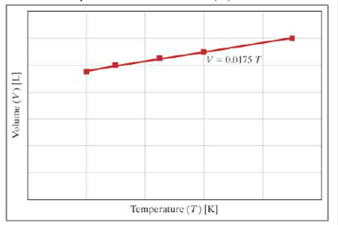
The graph shows the ideal gas law relationship (PV =nRT) between volume (V) and temperature (T).
- a. What are the units of the slope (0.0175)?
- b. If the tank has a pressure of 2.4 atmospheres and is filled with nitrogen (formula, N2; molecular weight, 28 grams per mole), what is the mass of gas in the tank in units of grams?
- c.
 If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
a.
Find the slope units.
Answer to Problem 1ICA
The units of slope is
Explanation of Solution
Calculation:
Refer to the graph in the respective question. The expression is,
Rearrange the expression.
From the graph, the unit of volume (V) is L and temperature (T) is K. Therefore, the unit of slope (0.0175) is
Conclusion:
Thus, the units of slope is
b.
Find the mass of gas in terms of grams.
Answer to Problem 1ICA
The mass of nitrogen gas in terms of grams is 14.4 g.
Explanation of Solution
Given data:
Molecular weight (MW) of nitrogen is 28 grams per mole.
Pressure (P)is 2.4 atmospheres.
Formula used:
Consider the ideal gas law relationship.
Here,
Calculation:
Refer to the graph in the respective question. The expression is,
Modify equation (1) as follows.
Substitute
Substitute 2.4 atm for P and
Consider the general expression for mass in terms of molecular weight.
Substitute
Conclusion:
Thus, the mass of nitrogen gas in terms of grams is 14.4 g.
c.
Find the pressure of tank in terms of atmospheres.
Answer to Problem 1ICA
The pressure of tank in terms of atmospheres is 3.0 atm.
Explanation of Solution
Given data:
Molecular weight (MW) of oxygen is 32 grams per mole.
Mass of oxygen (m)is 20 grams.
Calculation:
Refer to the graph in the respective question.
Modify equation (2).
Substitute 20 g for
Modify equation (1).
Substitute
Substitute 0.625 mol for n and
Conclusion:
Thus, the pressure of tank in terms of atmospheres is 3.0 atm.
Want to see more full solutions like this?
Chapter 12 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- 1. Suppose a person is 182 cm tall. A typical blood pressure is often given as 110/70 mm Hg, with a mean pressure of 80 mm Hg. A. Do you think this is the gauge pressure or absolute pressure? Why? 6, If the pressure at the top of the head is about 39 mm Hg, how far away is this person's head from the heart? Assume the density of blood is about 6% higher than that of water. C, What then would be the blood pressure of this person at their feet? D. Suppose this person suffers a puncture wound around the same height as the heart where the opening is about 3 mm in diameter. How far could blood shoot from their body assuming it comes out horizontally with 0 m/s initial horizontal velocity? Please show me how to solue thes problem iN nEat and clené de fail. Show ala equatiins that I must aSE fo Get neat I mUst correct asweres. 4o Ger he Thank gon !!arrow_forward2. The formula below finds the Reynold's number, Re, a measure of the characteristics of fluid flow in a pipe. Dvp a. If D (diameter of the pipe) is measured in meters, v (flow velocity) is measured in meters/second, pis density measured in kilograms/meter, and Re is dimensionless, what are the units for u, the dynamic viscosity? b. The dynamic viscosity of ethyl alcohol is 2.0 x 105 Ibf-s/ft?. Convert this to SI units you found in Part a.arrow_forwardThe pressure difference between 2 points is 100,000 Pascals of a fluid column with height of 4 meters. The specific gravity of the fluid is closest to what value? The answer has a margin of 0.05 from the exact answer.arrow_forward
- Study the table to the right and then answer these questions by filling in the blank columns in the table.1. Convert the miles per gallon figures in the table tokilometers per liter (kpl).2. How many liters (and how many gallons) of gasolinewould each type of car use annually if it were driven19,300 kilometers (12,000 miles) per year?3. How many kilograms (and how many pounds) of carbon dioxide would be released into the atmosphereannually by each car, based on the fuel consumptioncalculated in question 2? Assume that the combustion of gasoline releases 2.3 kilograms of CO2 per liter(19 pounds per gallon).arrow_forwardUse the 3 relations (equations) below to relate T(°F) to T(°C). Then, find out, at what temperature a Fahrenheit and a Celciusthermometer would read the same numerical value.arrow_forwardTime left 1:5 An ideal gas has its pressure (1.8) times and mass density (2.2) times increased. If the initial temperature is 265.4 °C, what is the final temperature in °C, and use one number after the decimal (xxx.x)? Answer: NEXT PAGE pe here to search DELLarrow_forward
- Two tanks are connected in the following unusual manner. F₁ Notes: h₂ F₂ J h₂ What are the algebraic equations & ODEs that can be used to find h₁, h₂, F₂, & F3 as functions of time for any given variations in inputs. b. Identify all input & output variables. The density of the incoming liquid, p, is constant. The cross-sectional areas of the two tanks are A and Ą. F₂ is positive for flow from Tank 1 to Tank 2. The two valves are linear with valve coefficients C₂ and C3. Both tanks are open to the atmosphere.arrow_forward. I am planning to perform some volume-flow rate measurements in the Fluid Mechanics Laboratory. For this, I need a volumetric measuring tank (graduated cylinder) and a stopwatch. I considered the volume of the measured tank as 15 gallons and a stopwatch with reaction time as 1/10th of a second (though resolution of 1/1000th of a second). What is the volume flow rate if it takes 5 minutes to fill a 15-gallon of tank? Determine the smallest division to be on the tank in order to estimate the volume flow rate within an accuracy of ± 0.05 gpm.arrow_forwardSHOW THE TABLE USED! A fixed amount of carbon dioxide goes through a polytropic process where n=1.4. The initial temperature of the CO2 is 250 K. The final temperature of the CO, is 400 K. If the mass of the CO2 is 12 kg and behaves as an ideal gas, determine the work, the change in internal energy, and the heat transfer during the process (in kJ). Note that PV=mRT. In this problem, can the carbon dioxide be considered an ideal gas at the initial conditions if the initial pressure is 150 kPa? Justify your answer quantitatively.arrow_forward
- In a hydraulics laboratory, a certain test liquid flows incompressible. For the flow of this liquid, it is correct to state that: Answers: a) The pressure must be the same at all points within the liquid. b) the velocity must be the same at all points within the liquid. c) the density must have the same value at all points within the liquid and cannot change as it flows. d) the pressure at a certain point within the liquid cannot change as it flows.arrow_forwardTHERMODYNAMICS UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS/DIAGRAMS LEGIBLY. NO LONG EXPLANATION NEEDED. USE 3 DECIMAL PLACES. BOX THE FINAL ANSWER. An airplane is cruising at an elevation of 35,000 feet from sea level. Determine the amount of gage pressure in bars needed to pressurize the airplane to simulate sea level conditions. The average atmospheric pressure on earth is mated as a function of altitude by the relation Patm = 101.325 (1- 0.02256z)5.256, where Patm is the atmospheric pressure in kPa and z is the altitude in km with z = 0 at sea level.arrow_forwardDwarf Fortress. A group of dwarves made an outpost far from their homeland, "Moria, Middle-Earth". They have set-up the outpost near the river. In order to attract adventurers to their outpost, they constructed an underground tavern below (height h1 = 50m) the river. They dug a tunnel and an artificial cistern. Just above = this cistern (height h2= 10m) is the underground tavern. Using Bernoulli's equation and Fluid dynamics, explain if this plan is going to work or not. Meaning, explain if the water will pour out of the cistern into the tavern. If this plan is not going to work, what will be your suggestions (Dwarves live underground. They refuse to create the tavern on the surface)arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





