
(a)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept Introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
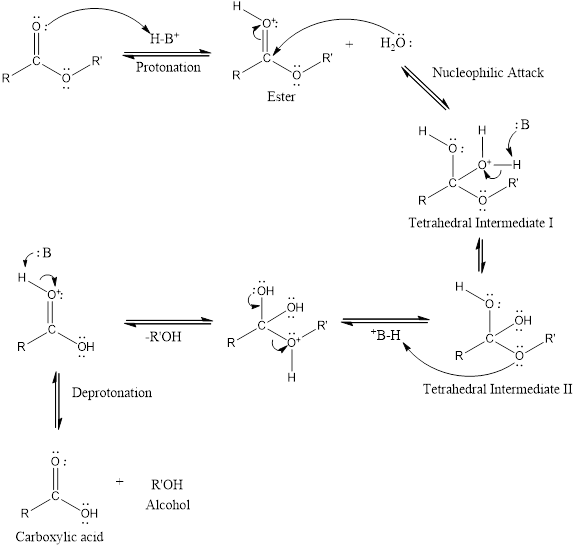
Therefore, products obtained by the acid catalyzed ester hydrolysis are the
(b)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
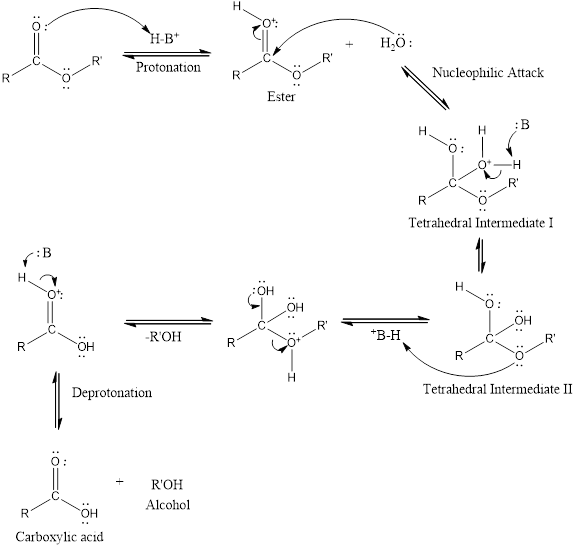
Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(c)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept Introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
- Propose a reaction for the formation of the following products involving ester formation.arrow_forwardHydration of aldehydes and ketones can be catalyzed by acid or base. Bases catalyze hydration by: protonating the carbonyl oxygen making the carbonyl group more electrophilic employing hydroxide ion, which is a better nucleophile than water making the carbonyl group less electrophilic shifting the equilibrium position of the reaction to favor productsarrow_forwardPrepare the following compounds starting from benzaldehyde and the appropriate ketone. Provide reactions for preparing the ketones starting from aromatic hydrocarbon compounds.arrow_forward
- Draw the structures of the products from the hydrolysis of each of the following with NaOHNaOH.arrow_forwardThe product of this reaction is a(n): H3CO OCH 3 cyanohydrin ketone aldehyde imine ester H3O+ productarrow_forwardWhich of these is the most viable route for the preparation or synthesis of a carboxylic acid? O Alpha-carbon substitution of ketones O Oxidation of aldehydes O Nucleophilic acyl substitution of amides O Elimination of alcohols O Nucleophilic addition of ketonesarrow_forward
- What explains why many aldehydes and ketones can undergo self-condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon is an electrophile. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile. The oxygen of the carbonyl group can attack the carbon of the carbonyl group. Only esters can undergo self-condensation reactions.arrow_forwardGive the reagents and intermediate products for the following two-step reaction.arrow_forwardName the carbonyl compound that would be formed by the complete acidic hydrolysis of the following hemiacetal/hemiketal or acetal/ketal: OH OCH₂CH₂CH₂CH₂CH3arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


