
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.5, Problem 8P
Using the pKa values listed in Table 11.1, predict the products of the following reactions:
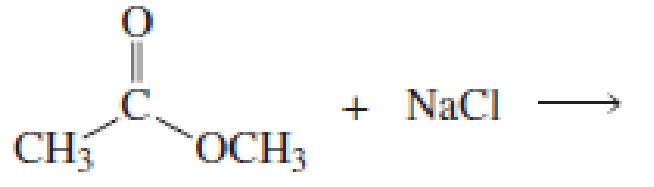
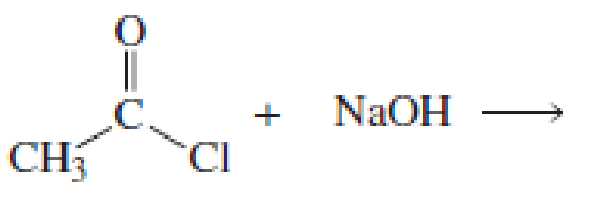
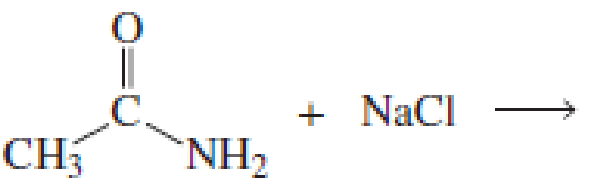
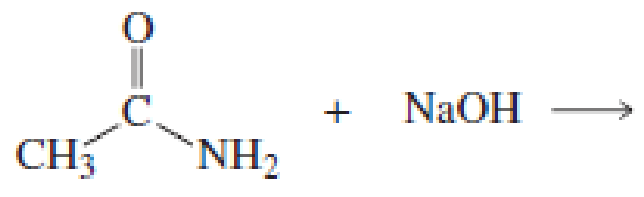
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the product of each of the following reactions:a) HClO4 (aq) + Fe2O3 (s) ===>b) H2SO4 (aq) + Sr (s) ===>c) H3PO4(aq) + KOH(aq) ===>
Maleic anhydride may be prepared using two routes:
Oxidation of benzene:
Oxidation of but-1-ene:
76
+30₂
+ 2COz + 2H,O
+ 3 H₂O
The benzene oxidation route typically occurs in 65 % yield, while the but-1-
ene route only gives yields of 55 %.
(a) Assuming that each reaction is performed in the gas phase only, and that
no additional chemicals are required, calculate (i) the atom economy and (ii)
the effective mass yield of both reactions. You should assume that O₂, CO₂
and H₂O are not toxic.
(b) Which route would you recommend to industry? Outline the factors which
might influence your decision.
How do you determine which reaction is thermodynamically favorable using pka values?
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound p-methoxybenzonitrile N-oxide, which has the formula CH3OC6H4CNO, reacts with itself to form a dimer—a molecule that consists of two p-methoxybenzonitrile N-oxide units connected to each other (CH3OC6H4CNO)2. The reaction can be represented as A + A → B or 2 A → B where A represents p-methoxybenzonitrile N-oxide and B represents the dimer, (CH3OC6H4CNO)2. For the reaction in carbon tetrachloride at 40 °C with an initial concentration of 0.011 M, these data were obtained: Determine the rate law for the reaction. Determine the rate constant. Determine the order of the reaction with respect to A.arrow_forwardBriefly explain or give an introduction to this. Structure Elucidation of Compounds using Several Spectroscopic Techniques. 1H and 13C NMR Spectroscopy IR Spectroscopy Mass Spectroscopyarrow_forwardCompound A reacts with hydrogen to form cyclooctane. Upon reaction with 03, compound A forms 2 moles of 20. Provide a structure for compound A.arrow_forward
- For the Reaction Scheme below provide the following (i) list all the reagents and conditions needed in each step to account for the formation of the product(s) shown below. Br 1) 2) 3)arrow_forwardA compound of unknown structure (Compound A) is determined by elemental analysis to have a molecular formula of C10H12. Upon reaction with H2 and Pd/CacO3 the unknown absorbs one mole equivalent of hydrogen gas. Upon reaction with ozone, followed by zinc/acetic acid a product with structure shown is obtained. Four possibilities are given for the structure of Compound A. Select the correct answer. HO,C A B A Both B and C are correctarrow_forwardExplain why the product distribution is observed for the following reactions:arrow_forward
- 08) The NMR spectra of the two isomeric compounds with formula C3H5ClO2 are shown in letters a and b. Low-field protons appearing in the NMR spectrum around 12.1 and 11.5 ppm, respectively, are shown highlighted. Draw the structures of the isomers.arrow_forward11. The reaction for the magnesium cation with 8-hydroxyquinoline is carried out in the presence of:A) Nitric acidB) Sodium hydroxideC) A solution of ammonia and ammonium chlorideD) Sulfuric acidE) Sodium acetatearrow_forward(b) Your task is to prepare styrene by one of the following reactions. Which reaction would you choose to give the better yield of styrene? Explain your answer. Br KOH EtOH, A (ii) KOH EtOH, Aarrow_forward
- Why is K2CO3 a good choice for deprotonation of benzoic acid? Which way does the equilibrium lie, and by how much? How would you determine the direction of the equilibrium without knowing the pka value? Thanksarrow_forwardA compound of unknown structure (Compound A) is determined by elemental analysis to have a molecular formula of C10H12. Upon reaction with H2 and Pd/CacO3 the unknown absorbs one mole equivalent of hydrogen gas. Upon reaction with ozone, followed by zinc/acetic acid a product with structure shown is obtained. Four possibilities are given for the structure of Compound A. Select the correct answer. HO,C B A D В Both B and C are correct O o o o oarrow_forwardWrite the structures of:(i) Ethanoic acid(ii) Hex analarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
What are CHNOPS? These Chemical Elements = 98% of Life | Biology | Biochemistry; Author: Socratica;https://www.youtube.com/watch?v=w90wFlR53VM;License: Standard YouTube License, CC-BY