
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.1, Problem 1P
The aromas of many flowers and fruits are due to esters such as those shown in this problem. What are the common names of these esters? (Also see Problem 41.)
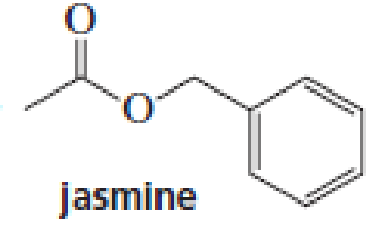
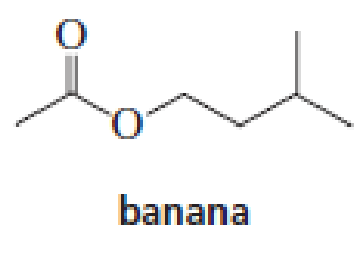
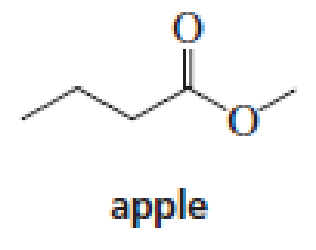
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hydration of aldehydes and ketones can be catalyzed by acid
or base. Bases catalyze hydration by:
protonating the carbonyl oxygen
making the carbonyl group more electrophilic
employing hydroxide ion, which is a better nucleophile than water
making the carbonyl group less electrophilic
shifting the equilibrium position of the reaction to favor products
What explains why many aldehydes and ketones can undergo self- condensation reactions in basic conditions?
The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon a an electrophile
O The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile
O The oxygen of the carbonyl group can attack the carbon of the carbonyl group
Only esters can undergo self-condensation reactions
4.
Aldehydes and ketones can be "protected" as acetals and ketals. Complete the following outlined
reactions, showing how the protection is accomplished, and what results after deprotection.
Br
MgBr
1)
Br
2) H3O*
deprotection here
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The major product that will form during the nitration of benzoic acid is? OA H₂N OR O-N ос OD. NO₂ COOH COOH COOR NH₂ COOH OZN H-N COOH COOHarrow_forwardWhich of these would likely take the LONGEST to hydrolyze (undergo hydralysis) to the carboxylic acid? 人 人 OH A. D. O All would hydrolyze rapidly B would be slowest O C would be slowest O D would be slowestarrow_forwardName the following carboxylic acid derivatives. a, b, c.arrow_forward
- What will be the major organic product formed in this reaction? но. OOH A) B) C) D) HO HO HO, Aarrow_forwardA mixture of the compounds shown below is dissolved in cyclohexane. An aqueous solution of NaOH is added to the solution, and two layers are formed. Which compound(s) is(are) found in the aqueous layer? он HO Benzoic acid Beta-naphthol Naphthalene O Benzoic acid only O Beta-naphthol only O Naphthalene O Both Benzoic acid and beta-naphthol O Both beta-naphthol and naphthalenearrow_forwardFollowing ester (methyl benzoate) was hydrolyzed in presence of an acid catalyst. This reaction produces --- and ---. OCH3 benzoic acid, ethanol benzoic acid, water acetic acid, benzene benzoic acid, methanolarrow_forward
- Complete the following reactions by filling in any missing reactants, reagents or products. a) Zn(Hg) conc. HCl b) ОН ? с) ОН РСС CH,Cl,arrow_forwardWith which of the following can ethyl ethanoate not undergo a substitution reaction? * a-aqueous NaOH b-CH3OH, H+ c-aqueous NH3 d-CH3COONaarrow_forward#16b. Provide the missing reactants, reagents, or products for the following reaction sequences below.arrow_forward
- What explains why many aldehydes and ketones can undergo self-condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon is an electrophile. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile. The oxygen of the carbonyl group can attack the carbon of the carbonyl group. Only esters can undergo self-condensation reactions.arrow_forwardQuestion 9 The reagent which converts a carbonyl group of a ketone into a enamine group is O CH3CH2CH2NHCH3; H CH3CH2CH2NH2; H* O CH3CH2CH2NHCH3; H3O* O CH3CH2CH2NH2; OH OLIAIH[OC(CH3)3]3arrow_forward20:24 : ●●● E) 03 12. Fill in the box with the major organic product of the first step of the reaction shown? The first step of the reaction shown is an exhaustive methylation and is one of the SN2, SN1, E2, or E1 reactions. Another piece of information about this full reaction, the second step proceeds via an E2 mechanism. xs CH₂l 13. What is the name of the reaction in the previous question? A) Swern Oxidation Hofmann Elimination ABCD Ag₂O, A Cope Elimination Williamson Ether Synthesis Send a chat Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY