
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 13EQ
This is done by removing an unshared pair from each of two adjacent atoms and adding one electron pair as a second bond between the atoms. Each such operation reduces the number of electrons in the trial structure by two. Removing the unshared pairs of electrons on the carbon atoms and adding a second carbon–carbon bond gives
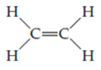
a structure in which there are four electrons involved in a double bond between the carbon atoms. The trial structure now contains electrons ____ and is correct.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of these is a hydroxyl group? A large organic molecule with different functional groups. A group marked A consists of a nitrogen atom and two hydrogen atoms attached to it. A group marked B consists of a phosphorus atom with four oxygen atoms. One of these oxygen atoms is connected to the phosphorus by a double bond. All other bonds are single. Two oxygen atoms have negative charges. A group marked C consists of a carbon atom connected to an oxygen atom by a double bond. A group marked D consists of an oxygen atom connected to a hydrogen atom. The group marked E consists of a sulfur atom connected with a hydrogen atom. A group marked F consists of a carbon atom, which is connected to one oxygen atom by a double bond and the other oxygen atom by a single bond. This last mentioned oxygen atom also has a hydrogen attached. EDACF
A. Structural Isomerism of Alkanes, continued.
Construct models for the five alkanes that have the molecular formula C6H₁4. All five of your models should have the same
number of each type of atom, but they should have the atoms connected in a different order. Thus the molecules they
represent are structural isomers of one another. Note the tetrahedral geometry of each carbon atom.
Draw an extended and a condensed structure (NOT skeletal/line angle) of each structural isomer, then determine its IUPAC
name. Recall that since the IUPAC name specifies the number of each type of atom and how they are connected, each
structural isomer will have a different name. Each name should very specifically describe the structure.
Isomer 3: Extended Structure
Isomer 3: Condensed Structure
IUPAC Name:
IUPAC Name:
Isomer 4: Extended Structure
Isomer 4: Condensed Structure
1. For each of the following compounds and ions, draw the complete Lewis structure. All formal charges should
be on the atoms that possess that formal charge.
CH4 H3CBr CH3CH₂OH H₂S H₂SO4 (sulfuric acid) H³0+ CH3*
2. Convert the following condensed formulas into bond-line structures, making sure to draw in zig-zag
formation.
CH3CH₂CH₂CH₂CH₂CH₂CH₂CH3
FCH₂CH₂I
H₂C=CHCH₂OH
(CH3)2CHCOOH
Chapter 1 Solutions
Pushing Electrons
Ch. 1 - 1. Hydrogen is a Group I element and each...Ch. 1 - Methanol has the molecular formula CH4O. Its...Ch. 1 - 3. The skeleton of chloromethane is...Ch. 1 - 4. Methanol’s skeleton is
Connecting all bonded...Ch. 1 - 5. The structure for chloromethane is
It...Ch. 1 - Prob. 6EQCh. 1 - 7. Dimethyl ether
No. of electrons in...Ch. 1 - Methylamine (CH5N) No. of electrons in structure...Ch. 1 - Methanethiol (CH4S) No. of electrons in structure...Ch. 1 - Methylal (C3H8O2) No. of electrons in structure...
Ch. 1 - Prob. 11EQCh. 1 - Adding electrons to the skeleton by making single...Ch. 1 - This is done by removing an unshared pair from...Ch. 1 - Prob. 14EQCh. 1 - Prob. 15EQCh. 1 - Prob. 16EQCh. 1 - The skeleton of acetyl chloride is . Write the...Ch. 1 - Three constitutional isomers exist for the formula...Ch. 1 - A number of constitutional isomers exist for the...Ch. 1 - Using the method outlined above, derive the...Ch. 1 - Prob. 21EQCh. 1 - Prob. 22EQCh. 1 - Prob. 23EQCh. 1 - Prob. 24EQCh. 1 - The skeleton of benzyldimethylamine is
The...Ch. 1 - The skeleton is benzaldoxime is The number of...Ch. 1 - Prob. 27EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 29EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 31EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - The Lewis structure of acetone is Circling the...Ch. 1 - Chloromethane has the Lewis...Ch. 1 - In the Lewis structure for chloromethane, the...Ch. 1 - Prob. 36EQCh. 1 - The oxygen atom in acetone possesses ____ unshared...Ch. 1 - Nitrobenzene has the skeleton
The number of...Ch. 1 - Prob. 39EQCh. 1 - Compute and add on the formal charges I these...Ch. 1 - Prob. 41EQCh. 1 - Prob. 42EQCh. 1 - Prob. 43EQCh. 1 - Prob. 44EQCh. 1 - Prob. 45EQCh. 1 - Prob. 46EQCh. 1 - Prob. 47EQCh. 1 - Compute and add on the formal charges in these...Ch. 1 - Prob. 49EQCh. 1 - Prob. 50EQCh. 1 - The n-propyl cation can be formed from a molecule...Ch. 1 - Prob. 52EQCh. 1 - Prob. 53EQCh. 1 - Methanol, CH3OH, is a compound in which the formal...Ch. 1 - When a proton becomes bonded to diethyl ether, by...Ch. 1 - Tetrahydrofuran has the structure
When a proton...Ch. 1 - Prob. 57EQCh. 1 - Prob. 58EQCh. 1 - The structure of pyridine is
When a proton...Ch. 1 - The carbon atom owns one electron from each of ...Ch. 1 - The n-butyl anion can be formed from When the CLi...Ch. 1 - The isobutyl anion can be formed from When the CNa...Ch. 1 - Prob. 63EQCh. 1 - Ethanol, , is a compound in which the formal...Ch. 1 - The loss of a proton attached to the oxygen atom...Ch. 1 - A very strong base can remove a proton from...Ch. 1 - Prob. 67EQCh. 1 - Prob. 68EQCh. 1 - Prob. 69EQCh. 1 - The homolysis of the OO bond in diacetyl peroxide...Ch. 1 - Prob. 71EQCh. 1 - Prob. 72EQCh. 1 - Prob. 73EQCh. 1 - Prob. 74EQCh. 1 - Prob. 75EQCh. 1 - Heterolytic cleavage of the CO bond to yield a...Ch. 1 - Prob. 77EQCh. 1 - Prob. 78EQCh. 1 - Prob. 79EQCh. 1 - Prob. 80EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Adding electrons to the skeleton by making single bonds between all bonded atoms gives Each hydrogen atom now has a pair of electrons, but each carbon has only 6 electrons. Adding a pair of electrons to each carbon gives the trial structure The number of electrons in the trial structure is ____. Since this number exceeds the number of available valence electrons, the structure is incorrect.arrow_forwardEthanol, , is a compound in which the formal charge on all the atoms is zero. Under certain conditions the bond can be broken so that both electrons remain with the oxygen atom. The products are In this structure the oxygen owns one electron from shared pair and two electrons from each of unshared pairs. The total number of electrons belonging to oxygen is Oxygen is a Group element. The formal charge on the oxygen atom is . The correct Lewis structure for the ethoxide ion is Note that the other fragment, the proton, leaves with a formal charge of +1.arrow_forwardSketch the hydrogen bond(s) that form between two molecules of ethanol and one nitrogen trihydride molecule and one dihydrogen monoxide molecule. Show the proper VSEPR shapes of all molecules (meaning VSEPR shapes with proper bond angles) and used dotted or dashed lines to indicate the hydrogen bonds. Indicate 4 different H-bonds: 1) ethanol and nitrogen trihydride 2) ethanol and dihydrogen monoxide 3) dihydrogen monoxide and nitrogen trihydride and then 4) between the two ethanol molecules.arrow_forward
- Bitumen (tar) is a mixture of various unsaturated (and also saturated) hydrocarbons of different chain lengths (it is used to make road pavement - asphalt). Based on your correlation, suggest a physical explanation of the fact that tar is black (i.e. absorbs light of all wavelengths).arrow_forwardWhat would the partially halogenated version of this molecule look like if it was the trans bond product and only one molecule of H2was added to solution?arrow_forwardWrite an equation for the reaction of CH3 SCH3 with BF3, a Lewis acid, and show by the use of curved arrows how the reaction occurs. • Show all hydrogen atoms that are not attached to a carbon atom. Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. • Use the "starting points" menu to revert to the original molecule(s) shown. • Draw the appropriate electron-flow arrows. • Omit+ signs between structures. ● ● CH3 | :S: | CH3 == starting points == ↑ TAYY : F کر ? ChemDoodleⓇarrow_forward
- complete a Carvone Lewis structure that displays all valence electrons, all atoms and all the formal charges, if any. Use only a single dash for each pair of bonding electrons and a pair of dots for all nonbonding pairs of electrons. Identify and label all functional groups (alcohols, amines, amides, carboxylic acids, ketones, aldehydes, aromatic rings, aromatic amines, etc) on the Lewis Structure. You do not have to circle alkanes. (Must be included in the preliminary submission and in the PowerPoint.)arrow_forward1. What is resonance theory? State five conclusionstgan can be drawn from the theory. 2.What factors confer aromaticity to an organic molecule. 3. What are the various ways by which alkenes can be synthesized. 4. State the two main experiment that were used to establish the extra stability of the benzene molecule.arrow_forwardWhat is Resonance Theory? Sate five conclusions that can be drawn from the theory. State the two main experiments that were used to establish the extra stability of the benzene molecule. What are the factors that confer Aromaticity to an organic molecule?arrow_forward
- A. Structural Isomerism of Alkanes Construct models for the five alkanes that have the molecular formula C6H14. All five of your models should have the same number of each type of atom, but they should have the atoms connected in a different order. Thus the molecules they represent are structural isomers of one another. Note the tetrahedral geometry of each carbon atom. Draw an extended and a condensed structure (NOT skeletal/line angle) of each structural isomer, then determine its IUPAC name. Recall that since the IUPAC name specifies the number of each type of atom and how they are connected, each structural isomer will have a different name. Each name should very specifically describe the structure. Isomer 1: Extended Structure Isomer 1: Condensed Structure IUPAC Name: IUPAC Name: Isomer 2: Extended Structure Isomer 2: Condensed Structurearrow_forwardBased on the models of bonding in ethylene and acetylene,which molecule should have the greater carbon—carbonbond strength?arrow_forwardNaming and Drawing Organic Molecules Comparing skeletal structures related by one fewer bond Could we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. Explanation Check MacBook Air G 1/5 Julianna 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License