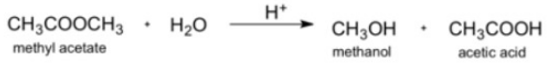
Metiyl acctate rcacts in acidic solution. CH,COOCH, • H30 CH,он . сн,соон н* methyl acetate methanol acesc acid The rale law is first order in melhyl acelale in acidic solutiva, aad the rate coaslant al 25°C is 1.26 x 10*/8. Ilow long will it take for 63.0 of the melhyl acelale lo react?
Metiyl acctate rcacts in acidic solution. CH,COOCH, • H30 CH,он . сн,соон н* methyl acetate methanol acesc acid The rale law is first order in melhyl acelale in acidic solutiva, aad the rate coaslant al 25°C is 1.26 x 10*/8. Ilow long will it take for 63.0 of the melhyl acelale lo react?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.42P
Related questions
Question

Transcribed Image Text:Metiyl acctate rcacts in acidic solution.
CH,COOCH,
• H30
CH,он . сн,соон
н*
methyl acetate
methanol
acesc acid
The rale law is first order in melhyl acelale in acidic solutiva, aad the rate coaslant al 25°C is 1.26 x 10*/8. Ilow long will it take for 63.0 of the melhyl acelale lo react?
Expert Solution
Step 1
Given:
This reaction is first order reaction.
Rate constant of the reaction, k = 1.26*10-4/s
Percentage of methyl acetate reacts = 63%
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning