Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter19: Nuclear Magnetic Resonance Spectroscopy
Section: Chapter Questions
Problem 19.15QAP: How will E for an isolated 13C nucleus compare with that of a 1H nucleus?
Related questions
Question

Transcribed Image Text:lonization rate is strong function of
Temperature
.1
number of free electrons
.2
Number of holes available
.3
Number of protons
.4
.avaiable
Expert Solution
Step 1
Ionization Rate (J) can be given by the realtion as-
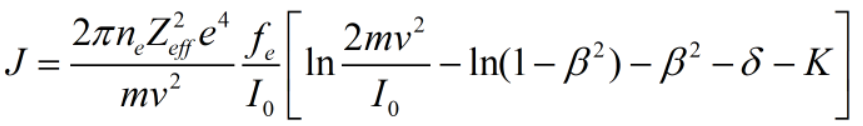
where,
fe: effective fraction.
v: velocity of incident particle.
I0: Ionization potential.
β: particle velocity.
δ: correction term.
K: constant
Zeff: effective charge.
ne: number of electrons.
m: mass of electron.
Step 2
From the ionization rate, it is clear that it is directly proportional to the number of electrons taking part during the reaction.
Therefore, Ionization Rate is strong function of number of electrons.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,