atoms Ħ Stomie meds Group fo Jaokat JK 132 318 34 19 Jaokat is ar iserught after metal Atá alundanas he Jk (3%), 3JK (20.0%), "Jk (50.0%.). She aug 317,12 amu 313 315 atomic mass is find the percent abundauce and the Ħ f nevtrons, pstens, and elecdsos left of tuor ineutid intepes 318 k and 319 JK?
atoms Ħ Stomie meds Group fo Jaokat JK 132 318 34 19 Jaokat is ar iserught after metal Atá alundanas he Jk (3%), 3JK (20.0%), "Jk (50.0%.). She aug 317,12 amu 313 315 atomic mass is find the percent abundauce and the Ħ f nevtrons, pstens, and elecdsos left of tuor ineutid intepes 318 k and 319 JK?
Related questions
Question

Transcribed Image Text:atomis #
Stomic mede
Group
nfo
Jaokat
JK
132
318 34
1.
Jaokat is ar isought after metsl , stá alundonas he
315 JK (20.0%), 3173K (50.0%.), he aug
313
atomic mass is
317,12 amu
find the percent abundouse andd the Ħof nevtions,
pysteps, and eledsone left of turo ineutid intopes
318 k and
319 JK?
Expert Solution
Step 1
The total percent abundance of an element would be equal to the sum of the percent abundances of all its isotopes.
Let x1, x2, x3, x4, and x5 be the percent abundances of the isotopes of JK.
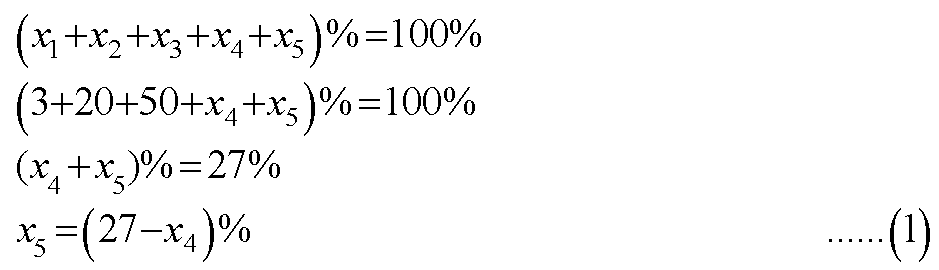
Let the atomic masses of the isotopes be A1, A2, A3, A4, and A5, respectively.
The average atomic mass of the element is given by the following equation.
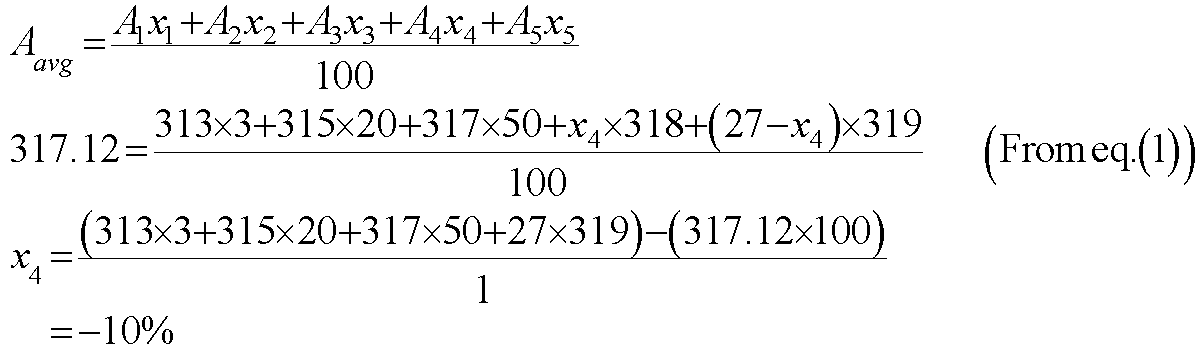
Substitute the value of x4 in equation (1) to get the value of x5 in the following way.
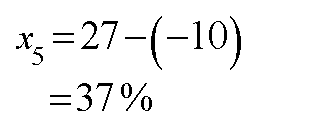
Note:
The percent abundance of 318JK is coming negative. This is not acceptable. Please verify your data.
Step by step
Solved in 2 steps with 5 images
