A gas sample enclosed in a rigid metal container at room temperature (20.0°C) has an absolute pressure p₁. The container is immersed in hot water until it warms to 40.0° C. What is the new absolute pressure på? Express your answer in terms of p1. View Available Hint(s) P2 = [-] ΑΣΦ Submit ?
A gas sample enclosed in a rigid metal container at room temperature (20.0°C) has an absolute pressure p₁. The container is immersed in hot water until it warms to 40.0° C. What is the new absolute pressure på? Express your answer in terms of p1. View Available Hint(s) P2 = [-] ΑΣΦ Submit ?
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter15: Thermodynamics
Section: Chapter Questions
Problem 12PE: Steam to drive an old—fashioned steam locomotive is supplied at a constant gauge pressure of...
Related questions
Question
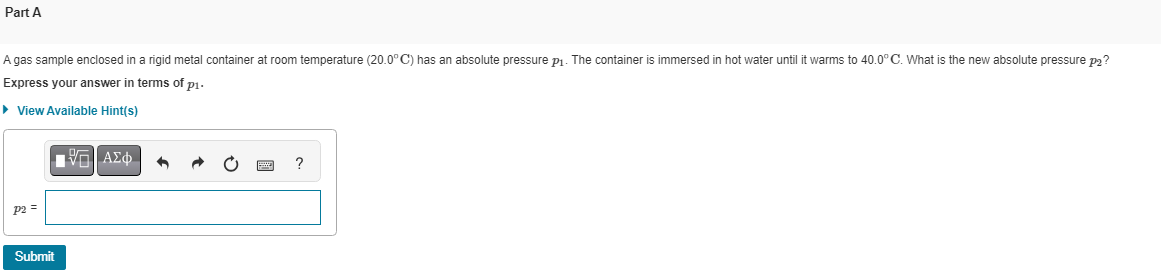
Transcribed Image Text:Part A
A gas sample enclosed in a rigid metal container at room temperature (20.0°C) has an absolute pressure p₁. The container is immersed in hot water until it warms to 40.0° C. What is the new absolute pressure p2?
Express your answer in terms of p1.
► View Available Hint(s)
P2 =
Submit
IVE ΑΣΦ
3
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
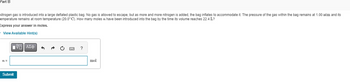
Transcribed Image Text:Part B
Nitrogen gas is introduced into a large deflated plastic bag. No gas is allowed to escape, but as more and more nitrogen is added, the bag inflates to accommodate it. The pressure of the gas within the bag remains at 1.00 atm and its
emperature remains t room temperature (20.0°C). How many moles ʼn have been introduced into the bag by the time its volume reaches 22.4 L?
Express your answer in moles.
View Available Hint(s)
n =
Submit
VE ΑΣΦ
mol
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
