
Concept explainers
Consider the following energy diagram.
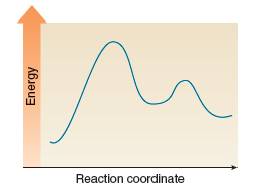
(a) How many steps are involved in this reaction?
(b) Label
(c) Label each transition state.
(d) Which point on the graph corresponds to a reactive intermediate?
(e) Which step is rate-determining?
(f) Is the overall reaction endothermic or exothermic?
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry (6th Edition)
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties
Organic Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Principles of Chemistry: A Molecular Approach (3rd Edition)
- 4. A) The enzyme urease catalyzes the hydrolysis of urea to ammonia and carbon dioxide. The uncatalyzed reaction has an activation energy of 125 kJ/mol. The enzyme catalyzes a mechanism that has an activation energy of 46 kJ/mol. By what factor does urease increase the rate of urea hydrolysis at 21 celsius? B) True or False: A catalyst increases the rate of a reaction by changing the mechanism of the reaction such that there is a different rate law for the reaction when a catalyst is present. C) True or False: A catalyst increases the equilibrium constant of a reaction and therefore makes the products more favored. D) True or False: In order for a catalyst to function it must exist in the same phase as the substrate.arrow_forwardDraw an energy diagram for a reaction in which the products are higher in energy than the starting materials and Ea is large. Clearly label all of the following on the diagram: the axes, the starting materials, the products, the transition state, ΔHo, and Ea.arrow_forwardConsider the following reaction: (a) The rate law for this reaction is first order in HBr(g) and first order in O₂(g). What is the rate law for this reaction? Rate = k [HBr(g)] [O₂(g)] O Rate = k [HBr(g)]² [O₂(g)] O Rate = k [HBr(g)] [O₂(g)]² O Rate = k [HBr(g)]² [0₂(g)]² O Rate = k [HBr(g)] [O₂(g)]³ O Rate = k [HBr(g)]4 [0₂(g)] (b) If the rate constant for this reaction at a certain temperature is 11500, what is the reaction rate when [HBr(g)] = 0.00379 M and [O₂(g)] = 0.00876 M? Rate = 4 HBr(g) + O₂(g) → 2 H₂O(g) + 2 Br₂(g) M/s. Rate = (c) What is the reaction rate when the concentration of HBr(g) is doubled, to 0.00758 M while the concentration of O₂(g) is 0.00876 M? M/Sarrow_forward
- Consider the following reaction: (a) The rate law for this reaction is first order in NO₂(g) and first order in O3(g). What is the rate law for this reaction? O Rate = k [NO₂(g)] [03(9)] Rate = k [NO₂(g)]² [03(9)] O Rate = k [NO₂(g)] [03(9)]² O Rate = k [NO₂(g)]² [03(g)]² Rate = k [NO₂(g)] [03(g)]³ Rate = k [NO₂(g)]4 [03(9)] (b) If the rate constant for this reaction at a certain temperature is 73200, what is the reaction rate when [NO₂(g)] = 0.973 M and [O3(9)] = 1.42 M? Rate = 2 NO₂(g) + 03(g) → N₂05(9) + O₂(g) M/s. Rate = (c) What is the reaction rate when the concentration of NO₂(g) is doubled, to 1.95 M while the concentration of O3(g) is 1.42 M? M/sarrow_forwardGiven the following balanced equation, determine the rate of reaction with respect to [H,]. (6HNZ - (6He + (0°N O Rate 2 4H2 O Rate # - O Rate O Rate = O tis not possible to determine the answer without more information.arrow_forward⦁ 4. Make your own energy diagram for an exothermic reaction that does not include a catalyst and one with a catalyst (Be sure each line is a different color or labeled). Label the axis of the diagram. Then, state what a catalyst is and how a catalyst affects the reaction rate.arrow_forward
- What type of reaction is this? In this diagram, what is the potential energy of C? In this diagram, what is the potential energy of D? In this diagram, what is the Eд without an enzyme? In this diagram, what is the Eд with an enzyme? In this diagram, what is the AG? 250 200 PE EA1 150 (kJ) D 100 "E с 50 Reaction pathwayarrow_forwardThe following reaction occurs in your car's exhaust system catalytic converter:Heat + 2CO + O2 + Pt → 2CO2 + PtWhat is the catalyst?arrow_forwardii) Explain the effect of Catalyst on the rate of a chemical reaction.arrow_forward
- Which of the following is true given the information below? 3 H₂ (g) + N₂ (g) → 2NH₃ (g); ∆H = -22 kcal/mol A) The reaction is endothermic. B) The product is higher in energy than the reactants. C) Heat is released D) The activation energy for the reaction is negative.arrow_forward2 Define "activation energy" a b What is the role of a catalyst in chemical reactions? Draw an energy profile diagram of an exothermic reaction and show the reaction pathway both with and without a catalyst present.arrow_forward(a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion. A balanced chemical reaction is necessary to relate the rate of reaction to the concentration of a reactant. As a reaction progresses its rate goes down. The rate of a slow step has more effect on the overall reaction rate than the rate of a fast step. Reaction rates are determined by reactant concentrations, temperatures, and reactant stabilities. Reaction rates increase with increasing temperature. Reactions involving very unstable combinations of chemicals have large rate constants.arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
