
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.11, Problem 21P
What hybrid orbitals are used to form the carbon-carbon
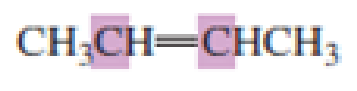


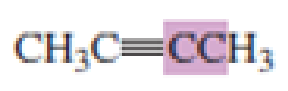

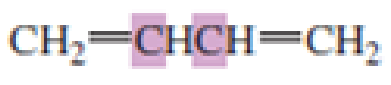

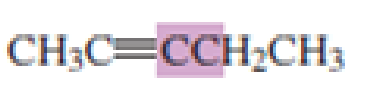
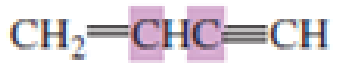
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the molecular geometry of the circled carbon?
НО,
ОН
Chemistry
In
enol form
Keto form
O
C6H5
C6H5
H
keto form
keto form
please show electron arrow.
OH
enol form
OH O
enol form
For the cation shown, four resonance structures are possible. Two resonance forms are given, but they are incomplete. Complete
structures 1 and 2 by adding nonbonding electrons and formal charges. Complete the two remaining resonance structures
according to their description, including nonbonding electrons and formal charges.
Structure 1: add lone pairs and charges
X
H₂C
H₂C
CH3
CH₂
Structure 2: add lone pairs and charges
H₂C
H₂C
0-
CH₂
CH₂
Chapter 6 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Label all carbons as C, CH, CH2, CH3 in the line structure of diosmin i.e, a flavonoid natural product and dietary supplement utilized to treat venous diseases or hemorrhoids. OH HO HO ÓH HO HO. ОН О ОН Diosminarrow_forwardWhich structure is NOT aromatic? CH CHarrow_forwardIn the ether given below, which carbon bonded to oxygen will have its bond cleaved when reacted with aqueous NaOH? Write the letter only.arrow_forward
- For each highlighted carbon identify what type of orbitals it has and how many of each. Then predict the approximate bond angle about each highlighted carbon.arrow_forwardWhat is the hybridization of the C in this aldehyde?arrow_forwardName the following ethers. Use -oxy if groups on either side of -O—are too complicated.arrow_forward
- Draw propyne and fill in all H’s .arrow_forwardFind the structures of the A-B-C compounds formed in the following reactions.arrow_forward3. Some students also confuse the structure of cyclohexanol with that of a phenol. Both structures have a six-carbon ring with a hydroxyl group attached. Use your learnings from the CHM 124 lecture course to explain how the six-carbon rings of cyclohexanol and phenol are different from one another.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License