
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 7P
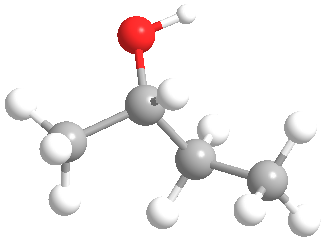
Does the molecular model shown represent
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1: Give at least five (5) uses of Alcohol and Phenol
Name
Functional Group
R-OH
Alcohols
LOH
Phenols
Based on the illustration above. What is the difference between
alcohol and phenol?
Can Phenol react with alcohol?
When trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanoneWrite the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide Why doesn’t the cis isomer yield the oxide?Write the mechanism for each of the two reactions. .
Draw the product of the reaction between a ketone and an alcohol. Include all hydrogen atoms in the product.
རྐང་པའི
H*
+1 mole equiv.
CH, HỌ CH, CH
Select
Draw
Rings
More
G
c
Q2Q
Erase
How would you classify the product of the reaction? Note that a hemiacetal formed from a ketone is also called a
hemiketal; an acetal formed from a ketone is also called a ketal.
00
The product is a ketone.
The product is a hemiketal.
The product is an alcohol.
The product is a ketal.
Chapter 4 Solutions
Organic Chemistry - Standalone book
Ch. 4.2 - Examine the following for chirality centers:Ch. 4.2 - Prob. 2PCh. 4.3 - Prob. 3PCh. 4.3 - Prob. 4PCh. 4.4 - Prob. 5PCh. 4.4 - Prob. 6PCh. 4.5 - Does the molecular model shown represent...Ch. 4.6 - Assign absolute configurations as R or S to each...Ch. 4.6 - Draw three-dimensional representations ofCh. 4.7 - Prob. 10P
Ch. 4.7 - Using the Fischer projection of (R)-2-butanol...Ch. 4.8 - Prob. 12PCh. 4.9 - Prob. 13PCh. 4.9 - Prob. 14PCh. 4.10 - Prob. 15PCh. 4.10 - Draw Fischer projections of the four...Ch. 4.10 - Prob. 17PCh. 4.11 - A meso stereoisomer is possible for one of the...Ch. 4.11 - One of the stereoisomers of...Ch. 4.12 - Prob. 20PCh. 4.12 - Prob. 21PCh. 4.13 - Prob. 22PCh. 4.13 - Prob. 23PCh. 4.13 - Prob. 24PCh. 4.14 - Prob. 25PCh. 4 - Prob. 26PCh. 4 - Including stereoisomers, write structural formulas...Ch. 4 - Prob. 28PCh. 4 - Prob. 29PCh. 4 - Prob. 30PCh. 4 - Prob. 31PCh. 4 - Prob. 32PCh. 4 - Prob. 33PCh. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Prob. 38PCh. 4 - Prob. 39PCh. 4 - (-)-Menthol is the most stable stereoisomer of...Ch. 4 - Prob. 41PCh. 4 - Prob. 42PCh. 4 - (a) An aqueous solution containing 10 g of...Ch. 4 - Prob. 44DSPCh. 4 - Prob. 45DSPCh. 4 - Consider two chemical changes: one occurring at a...Ch. 4 - Consider two chemical changes: one occurring at a...Ch. 4 - Prob. 48DSPCh. 4 - Consider two chemical changes: one occurring at a...Ch. 4 - Consider two chemical changes: one occurring at a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following compounds is a carboxylic acid? O || CH3CH3CNH2 O || CH3CH3CCH3 O || CH3CH2COH O || CH3CH3COCH3 O || CH3CH3CHarrow_forward1-propanol + hydrobromic acid →arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone i. Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide. ii. Why doesn’t the cis isomer yield the oxide?. iii. Write the mechanism for each of the two reactions.arrow_forward
- Predict the IUPAC Product name. (Hint: first sketch the product then name choosing the correct IUPAC PRODUCT NAME BELOW). OH + NaOH sodium propanol sodium butanoate sodium propanoate O sodium butanalone sodium benzoate sodium propanal sodium butanolarrow_forwardCan an aldehyde have molecular formula C 5H 12O? Explain why or why not.arrow_forward1. What letter has an aldehyde with molecular formula C₄H₈O. 2. What letter has an ester with molecular formula C₄H₈O₂ 3. What letter has a ketone with molecular formula C₄H₈O₂ 4. What letter has a carboxylic acid with molecular formula C₄H₈O₂arrow_forward
- 2,2 Write down the products produced when the dimethyl-1-propanol compound is subjected to an acid-catalyzed water removal reaction.arrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H100) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCI) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardIn the chemical reaction shown below, 0.0100 mol of 2-methyl-2-propanol is reacted with 0.0175 mol of ammonium bromide and 0.0350 mol of ammonium chloride in the presence of 0.100 mol of sulfuric acid. What are the products of this reaction and their respective ratio to one another? OH respectively) respectively) 2-methyl-2-propanol 2-bromo-2-methylpropane and 2-chloro-2-methylpropane (ratio: 1:2; NH₂*Cr NH₂ Br respectively) H₂SO4 H₂O 2-bromo-2-methylpropane and 2-chloro-2-methylpropane (ratio: 1:1; ? + ? respectively) 2-bromo-2-methylpropane and 2-chloro-2-methylpropane (ratio: 2:1; 1-bromo-2-methylpropane and 1-chloro-2-methylpropane (ratio: 1:2;arrow_forward
- 3) Which one(s) of the following is/are an acetal ?arrow_forwardDraw 2‑methylpropanal. Include all hydrogen atoms. Predict the products when cyclohexanol is heated in the presence of H+.H+. Show all hydrogen atoms.arrow_forwardDraw seven constitutional isomers with molecular formula C3H6O2 that contain a carbonyl group. Identify the functional group(s) in each isomer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY