
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.5, Problem 3.31P
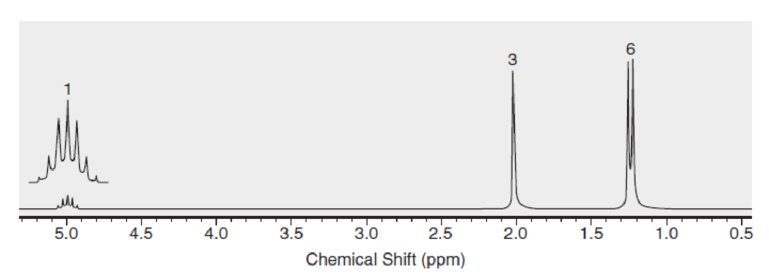
Below are NMR spectra of several compounds. Identify whether these compounds are likely to contain ethyl, isopropyl, and/or tert-butyl groups:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The 'H NMR spectrum of compound A (C3H100) has four signals: a multiplet at 8 = 7.25-7.32 ppm (5 H), a singlet at d = 5.17 ppm (1 H), a quartet at d
= 4.98 ppm (1 H), and a doublet at ô = 1.49 ppm (3 H). There are 6 signals in its 13C NMR spectrum. The IR spectrum has a broad absorption in the
-3200 cm-1 region. Compound A reacts with KMNO4 in a basic solution followed by acidification to give compound B with the molecular formula
C7H6O2. Draw structures for compounds A and B.
There are four esters with molecular formula C4H8O2. How can they be distinguished by 1H NMR?
Compound 2 has molecular formula C6H12. It shows three signals in the 1H-NMR spectrum, one at 0.96 ppm, one at 2.03 ppm, and one at 5.33 ppm. The relative integrals of these three signals are 3, 2, and 1, respectively.
Provide structure for compound 2, explain how you reached your conclusion.
Chapter 3 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
110. Have each group member choose a set of quantum numbers for an electron in a hydrogen atom. Calculate the w...
Chemistry: Structure and Properties (2nd Edition)
For the 3pz and 4dxz hydrogen-like atomic orbitals, sketch the following: a. The radial function R b. The radia...
Inorganic Chemistry
Two curves for the same reaction are shown in the following reaction energy diagram. Which curve represents the...
General, Organic, and Biological Chemistry (3rd Edition)
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
A circular tube of diameter D=0.2mm and length L=100mm in1poes a constant heat flux of qn=20103W/m2 on a fluid ...
Fundamentals of Heat and Mass Transfer
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of all constitutional isomers that contain a ketone and have molecular formula C5H10O. Give the IUPAC name for each isomer and state how 13C NMR spectroscopy could be used to distinguish these isomers.arrow_forwardA compound with molecular formula C3H8O produces a broad signal between 3200 and 3600 cm1 in its IR spectrum and produces two signals in its 1°C NMR spectrum. Draw the structure of the compound.arrow_forwardCompound B has molecular formula C9H12. It shows five signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.22 ppm, a septet of integral 1 at 2.86 ppm, a singlet of integral 1 at 5.34 ppm, a doublet of integral 2 at 6.70 ppm, and a doublet of integral 2 at 7.03 ppm. The 13C-NMR spectrum of B shows six unique signals (23.9, 34.0, 115.7, 128.7, 148.9, and 157.4). Identify B and explain your reasoning.arrow_forward
- Draw the structure of a compound with the molecular formula C8H₁0 that exhibits five signals in its ¹3C NMR spectrum, four of which appear between 100 and 150 ppm.arrow_forwardYou have a sample of a compound of molecular formula C11H15NO2, which has a benzene ring substituted by two groups, (CH3)2N− and −CO2CH2CH3, and exhibits the given 13C NMR. What disubstituted benzene isomer corresponds to these 13C data?arrow_forwardCompound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C-NMR shows five signals. The mass spectrum of A shows a peak at m/z = 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoning Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoning Compound B is treated with sodium ethoxide to generate compound C. The 1H-NMR spectrum of C shows…arrow_forward
- The H1H1 NMR spectra corresponds to an alcohol with the molecular formula C5H12O. Deduce the structure from the spectraarrow_forwardEthyl benzoate PhCO₂Et has these peaks in its 13C NMR spectrum: 17.3, 61.1, 100- 150 (four peaks) and 166.8 ppm. Which peak belongs to which carbon atom? Draw the structure of ethyl benzoate and clearly identify each carbon with its corresponding chemical shift.arrow_forwardHow could you distinguish the 1H NMR spectra of the following compounds?arrow_forward
- Compound A, with molecular formula C4H9Cl, shows two signals in its 13C NMR spectrum. Compound B, an isomer of compound A, shows foursignals, and in the proton-coupled mode, the signal farthest downfield is a doublet. Identify compounds A and B.arrow_forwardFollowing are 1H-NMR spectra for compounds G, H, and I, each with the molecular formula C5H12O. Each is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas. (a) Propose structural formulas of compounds G, H, and I. (b) Explain why there are four lines between 0.86 and 0.90 for compound G. (c) Explain why the 2H multiplets at 1.5 and 3.5 for compound H are so complex.arrow_forwardPropose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY