
DRAW IT Ø Label a hydrogen bond and a polar covalent bond in the diagram of live water molecules. Is a hydrogen bond a covalent bond? Explain.
To label: The hydrogen bond and covalent bonds in water molecules.
Introduction:
Water is a polar molecule consisting of an oxygen atom and two hydrogen atoms.
Explanation of Solution
Water (H2O) consists of an oxygen atom and two hydrogen atoms. Hydrogen and oxygen share their valence electrons to form a strong bond that known as a covalent bond (Fig.1). As oxygen is more electronegative than hydrogen, the shared electrons in the H-O bond tend to be pulled towards the oxygen atom. There are two regions of partial negative charge on oxygen and a partial positive charge on each hydrogen atom. Thus, the covalent bond between H-O in the water molecule is a polar bond.
Water is a polar molecule due to the difference in electronegativity between O and H atoms. The partial negatively charged oxygen atom of a water molecule is attracted to the partial positively charged hydrogen atom of an adjacent water molecule (Fig.1). This forms the hydrogen bond among different water molecules.
Pictorial representation:
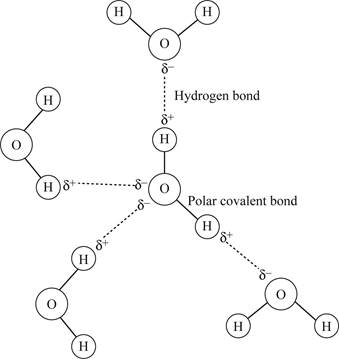
Fig. 1 Interactions among water molecules
To explain: The hydrogen bonds are not covalent bonds.
Introduction: Covalent bonds are formed by the sharing of valence electrons between two atoms. Hydrogen bonds are formed due to the attraction between two adjacent atoms due to partial charges.
Explanation of Solution
Covalent bonds are strong bonds, formed by the sharing of electrons between atoms. Hydrogen bonds do not involve the sharing of valence electrons. They are formed on the basis of attraction due to partial charges on neighboring atoms, and are hence very weak bonds. Therefore, a hydrogen bond is not a covalent bond.
Want to see more full solutions like this?
Chapter 3 Solutions
Campbell Biology
Additional Science Textbook Solutions
Human Biology: Concepts and Current Issues
Essentials of Genetics (9th Edition) - Standalone book
Genetics: Analysis and Principles
Biological Science (6th Edition)
Fundamentals of Anatomy & Physiology Plus Mastering A&P with eText - Access Card Package (10th Edition) (New A&P Titles by Ric Martini and Judi Nath)
- Figure 2.24 Which of the following statements is false? Molecules with the formulas CH3CH2COOH and C3H6O2 could be structural isomers. Molecules must have a double bond to be cis-trans isomers. To be enantiomers, a molecule must have at least three different atoms or groups connected to a central carbon. To be enantiomers, a molecule must have at least four different atoms or groups connected to a central carbon.arrow_forwardAn ________ can help keep the pH of a solution stable. a. covalent bond c. buffer b. hydrogen bond d. free radicalarrow_forwardWatch this video (http://openstaxcollege.org/l/disaccharide) to observe the formation of a disaccharide. What happens when water encounters a glycosidic bond?arrow_forward
- What kinds of bonds often control the shape (or tertiary form) of large molecules such as proteins? a. hydrogen b. ionic c. covalent d. inert e. singlearrow_forwardRefer to figure 2.15 to draw a picture of on NaCl molecule interacting with several water molecules. Label all molecules and ions appropriately and, as usual, use dotted lines to represent any hydrogen bonds.arrow_forwardWhy do you think surfactants denature proteins? Select all that apply. disulfide bonds can be created |disulfide bonds can be broken electrostatic bonds are formed electrostatic bonds are broken hydrophilic regions are enhanced hydrophobic regions are disrupted van der waals bonds are enhanced van der waals bonds are disrupted hydrogen bonds are enhanced hydrogen bonds are disruptedarrow_forward
- Picture below showing four water molecules interacting via hydrogen bonds? be sure lo label partial charges in each molecule and use dotted line to represent a hydrogen bond.arrow_forwardWhy is it unlikely that two neighboring water moleculeswould be arranged like this?H HH HO Oarrow_forwardWhich of the following is responsible for water exhibiting properties such as adhesion, co- hesion, and a high surface tension? A B с D covalent bonds between the constituent atoms presence of oxygen in the water molecule shape of the water molecule hydrogen bondingarrow_forward
- Explain in detail why water molecules are polar. Your explanation can be as long as you wish. Your answer must be in your own words. Your answer must include the following terms: Polar, polar covalent bonds, electronegativity, oxygen, hydrogen, partial positive, partial negartive, electrons.arrow_forwardfill the following table polymer or large biological molecule monomer or smaller subunit one funtion name od covalent bond nulceic acids three fatty acids easter bond acts as an enzyme immediate or long-term energy source glycosidic linkage explain the chemical reaction that occurs water molecules dissociate and reform. why is these chemical reactions the basis of the pH scale?arrow_forwardWhich of the following is not a characteristic of water due to hydrogen bonding? Solvent Adhesion Cohesion Adenosine O O Oarrow_forward

 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College



