
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.5, Problem 2.9P
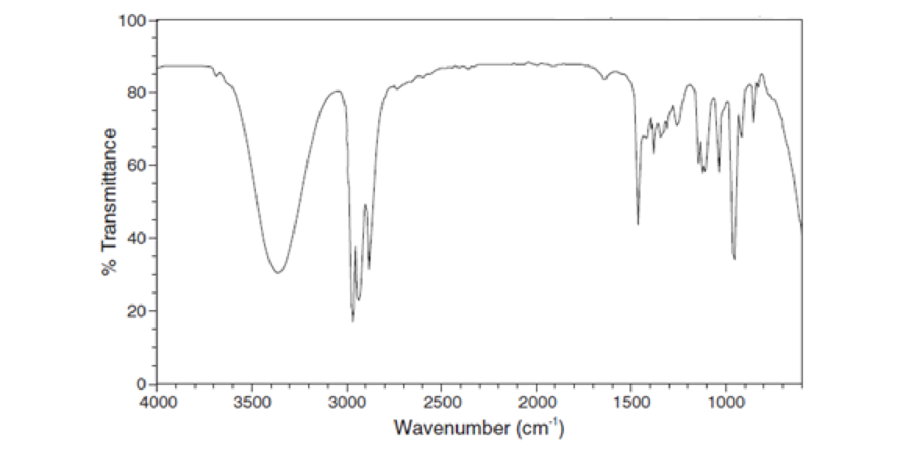
For each IR spectrum below, identify whether it is consistent with the structure of an alcohol, a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q-2.
Look at the following "C-NMR spectrum (Lower) and two DEPT spectra (C,H,CI):
131 ppm
140 130 120 110 100 90 80 70 60 s0 40 30 20 10 ppm
Label all the signals as CH,, CH,. CH or C. Put you labels in the lower
spectrum.
i)
ii)
Draw one possible structure for the compound.
5. Place the letter of the spectrum next to the name of the compound that it represents.
Compound
Letter
Acetone
1,2-dichloroethane
1,1,2-trichloroethane
2,2-dimethoxypropane
1-bromopropane
2-bromopropane
What are the major IR absorptions in the functional group region for each compound?
Chapter 2 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
37. Balance each redox reaction occurring in acidic aqueous solution.
a. K(s) + Cr3+(aq) → Cr(s) + K+(aq)
b. Al...
Chemistry: A Molecular Approach
Comprehend the variation of trapped gas pressure with volume. Concept Introduction: The volume is defined as th...
Living by Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
The cations and anions should be described. Concept introduction: An atom or group of atoms that possess charge...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please solve this organic chemistry question A carboxylic acid with the molecular formula C5H10O2 is treated with thionyl chloride to give compound A. Compound A has only one signal in its 1H NMR spectrum. Please figure out this carboxylic acid. You can write out name directly or molecular formula with functional groups (e.g. isopropanol (CH3)3COH). Hint: Only one H NMR signal means all hydrogens are in the same chemical status.arrow_forwardWhat is this compound base on the IR spectrum?arrow_forward3. a) Draw the below structure and answer the following questions. CI CH2COCH2CH3 i. Identify the chemically equivalent protons in the structure. Explain your answer. ii. Mention how many peaks you would expect in the HNMR spectrum of the compound. Explain the position of each peak in the spectrum. iii. Mention what would be the splitting pattern of each peak with proper explanation. iv. Draw the HNMR spectrum of the compound. b) Explain why the delta/ ppm scale was introduced instead of Hz in NMR analysis with example.arrow_forward
- True or False 1. A molecule that is "IR inactive" means that it does not produce any signal due to no vibration. 2. Infrared spectroscopic data is reported in wavenumber (cm-1) against absorbance because they have a linear relationship. 3. The signals observed from a molecule of chloropropane will have a higher wavenumber than iodopropane. 4. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than the C-C bond in an alkyne.arrow_forwardBelow are three MS spectra, Spectra 1, 2, and 3. Each of this mass spectrum corresponds to either Compound A (contains one Cl in the molecular formula), Compound B (one I in the molecular formula), and Compound C (one Br in the molecular formula). Identify which spectra corresponds to which compound. Provide explanations for your choices.arrow_forwardWhich one of the pi bonds in the molecules shown below will produce a stronger signal in an IR spectrum? Yes, compound I produces a stronger signal because it has a larger dipole moment. O Yes, compound II produces a stronger signal because it has a larger dipole moment. No, the alkene groups produce similar signals because they have similar double bonds. O No, the alkene groups produce similar signals because they both contain a similar number of atoms.arrow_forward
- The DEPT-90 spectrum exhibits 6 in the 0-50 ppm region The DEPT-135 spectrum exhibits x 100 ppm region that is a positive ▾ C6 signal(s) for the CH groups: ▼ 1,2,6 ✓ in the sp2 hybridized region 100-150 C3 and C4 ▼ signal(s) (only the quaternary carbon atoms, signal(s), indicating the presence of a methylene group (CH₂) attached to an oxygen atom, are missing); there is C5 ▼ C1 and C2 ▼ and signal(s) in the 50-arrow_forwardUsing the Molecular Ion to Identify a Compound Pent-1-ene and pent-1-yne are low-boiling hydrocarbons that have different molecular ions in their mass spectra. Match each hydrocarbon to its mass spectrum.arrow_forwardEach of the following IR spectra (shown in the picture, a-f) corresponds to one of the five isomers of C4H80. Match the spectrum to the correct structure and stated the reason.arrow_forward
- ANswer the question below explain and answer properly please which of these structures do not agree with the IR spectrum data (C=O, C-H) below?arrow_forwardIdentify the functional group of the molecule in the following IR spectrum.arrow_forwardAcetyleugenol: Match the peaks to the appropriate number on the structure. A letter may correspond to more than one number. 10 11 2. 2 3. 3 44 5. 5 6. 6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY