
Concept explainers
Write the chemical formula and Lewis structure of the following each of which contains five carbon atoms:
(a) an
(b) an
(c) an
a)
Interpretation:
The chemical formula and Lewis structure of an alkane with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The completely saturated hydrocarbon is known as an alkane.
The general molecular formula of alkane is
Answer to Problem 1E
Chemical formula:
Lewis structure:
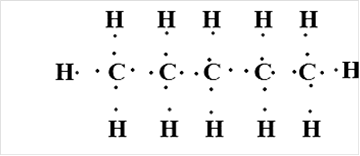
Pentane
Explanation of Solution
The general formula of alkane is
In case of five carbon atoms, the molecular formula of alkane is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 32
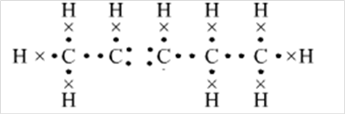
Thus, Lewis structure of alkane having five carbon atoms is:
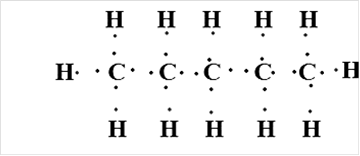 Or,
Or, 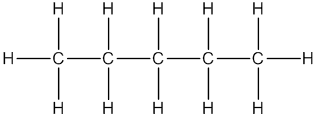
b)
Interpretation:
The chemical formula and Lewis structure of an alkene with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The unsaturated hydrocarbon with one or more double bond is known as an alkene.
The general molecular formula of alkene is
Answer to Problem 1E
Chemical formula:
Lewis structure:
Explanation of Solution
The general formula of alkene is
In case of five carbon atoms, the molecular formula of alkene is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 30
Thus, Lewis structure of alkene having five carbon atoms is:
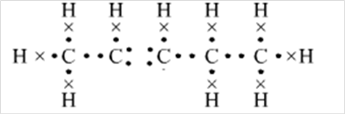 Or,
Or, 
c)
Interpretation:
The chemical formula and Lewis structure of an alkyne with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms.Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The unsaturated hydrocarbon with one or more triple bond is known as an alkyne.
The general molecular formula of alkyne is
Answer to Problem 1E
Chemical formula:
Lewis structure:
Explanation of Solution
The general formula of alkyne is
In case of five carbon atoms, the molecular formula of alkyne is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 28
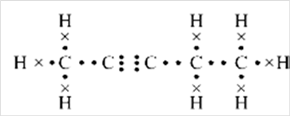
Thus, Lewis structure of alkyne having five carbon atoms is:
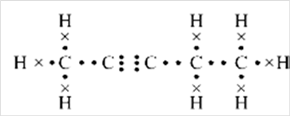 Or,
Or, 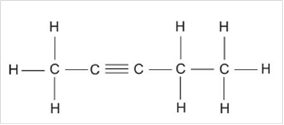
Want to see more full solutions like this?
Chapter 20 Solutions
Chemistry by OpenStax (2015-05-04)
Additional Science Textbook Solutions
College Physics
Chemistry: The Central Science (14th Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: A Molecular Approach
Introductory Chemistry (5th Edition) (Standalone Book)
General, Organic, and Biological Chemistry (3rd Edition)
- (a) What structural feature is associated with each type of hydrocarbon: alkane, cycloalkane, alkene, and alkyne?(b) Give the general formula for each type.(c) Which hydrocarbons are considered saturated?arrow_forwardAn alkane, P, has the molecular formula, C,H.. An alkene, Q, has the molecular formula, C H,. (a) Name P and Q ánd write their full structural formulae. (b) State two differences between P and Q in terms of their structures. x'arrow_forward(a) When the metallic element sodium combines with the nonmetallic element bromine, Br2(l), how can you determine the chemical formula of the product? How do you know whether the product is a solid, liquid, or gas at room temperature? Write the balanced chemical equation for the reaction. (b) When a hydrocarbon burns in air, what reactant besides the hydrocarbon is involved in the reaction? What products are formed? Write a balanced chemical equation for the combustion of benzene C6H6(l), in air.arrow_forward
- Organic chemistry is currently defined as(A) the study of compounds made only by livingcells.(B) the study of carbon compounds.(C) the study of natural (as opposed to synthetic)compounds.(D) the study of hydrocarbons.arrow_forwardWrite two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.(a) 2-butene reacts with chlorine.(b) benzene burns in air.arrow_forwardWhat structural features help us identify a compound as(a) an alkane, (b) a cycloalkane, (c) an alkene, (d) an alkyne,(e) a saturated hydrocarbon, (f) an aromatic hydrocarbon?arrow_forward
- (a) What is the difference between chlorofluorocarbons and hydrofluorocarbons?arrow_forward(a) Calculate the standard enthalpy change for the combustion of 1 mol of benzene, C6H61l2, to CO21g2 and H2O1l2.(b) Compare the quantity of heat produced by combustion of 1.00 g propane with that produced by 1.00 g benzene.arrow_forward(a) The compound given below had the following IUPAC name and structural formula dibromocyclopentane C3H6CHBrCHBr (i) What type of isomerism is possible in the organic compound? (ii) Draw all the pairs of possible isomers and name them.arrow_forward
- (a) (b) Define the terms 'functional group' and 'unsaturated hydrocarbon'. Name the following organic compounds: i. ii. (c) Draw skeleton structures for the following organic compounds: i. 3,4-dimethylheptane 3-methylheptanal ii. OH (d) The following two organic compounds are structural isomers to each other. Carefully identify and justify the structural isomers type (skeletal, functional, or positional) with their common molecular formula. HO O HO nos. A Barrow_forward(a) What is a functional group? (b) What functional groupcharacterizes an alcohol? (c) Write a structural formula for1-pentanol, the alcohol derived from pentane by making asubstitution on one of the carbon atoms.arrow_forwardMTBE, Methyl tert-butyl ether, CH3OC(CH3)3, is used as an oxygen source in oxygenated gasolines. MTBE is manufactured by reacting 2-methylpropene with methanol.(a) Using Lewis structures, write the chemical equation representing the reaction.(b) What volume of methanol, density 0.7915 g/mL, is required to produce exactly 1000 kg of MTBE, assuming a 100% yield?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
