
Concept explainers
PRACTICE PROBLEM 15.1
Show how loss of a proton can be represented using each of the three resonance structures for the arenium ion and show how each representation leads to the formation of a benzene ring with three alternating double bonds (i.e., six fully delocalized
Interpretation:
The loss of protons from three resonating structures of the areniumion, are to be shown.
Concept Introduction:
Arrows present in the mechanism of the reaction indicate the electron transfer, starting from negatively charged species and ending at the positively charged species.
Electrophiles attract negatively charged species and nucleophiles attract positively charged species.
Answer to Problem 1PP
Solution: The loss of protons from the three resonating structures of the arenium ion are shown as follows:
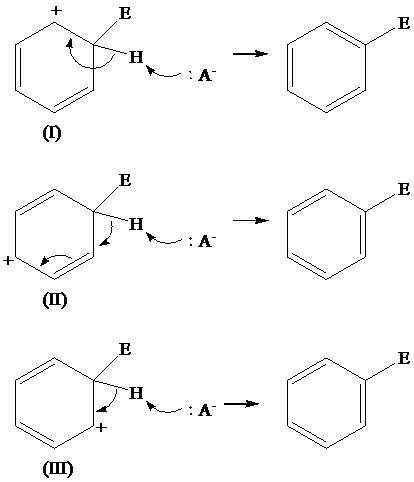
Explanation of Solution
The resonating structures of the arenium ion, formed in the electrophilic substitution reaction of benzene, are shown below:

The removal of proton occurs from the carbon atom where the electrophile is attached and the bond pairs of electrons of
The removal of proton from (I) resonating structure is shown as follows:
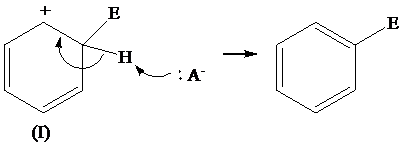
The removal of proton from (II) resonating structure is shown as follows:
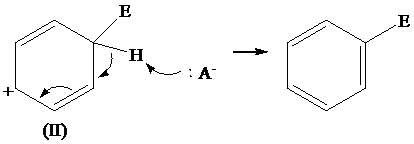
The removal of proton from (III) resonating structure is shown as follows:
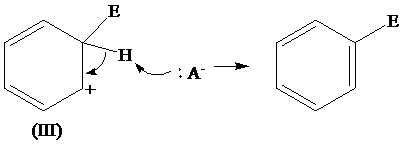
The removal of protons from the arenium ion leads to the transfer of bond pairs to the benzene ring and the delocalization of these electrons on the ring occurs, to form the final product.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Chemistry & Chemical Reactivity
Organic Chemistry (9th Edition)
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
- Compound Y (molecular formula C6H10) gives four lines in its 13C NMRspectrum (27, 30, 67, and 93 ppm) and the IR spectrum given here.Propose a structure for Y. Additional spectroscopy problems on alkynes are given in Chapters B and C:Infrared spectroscopy: B.4a; B.5; B.16a; B.19a; B.21a, d; B.29Nuclear magnetic resonance spectroscopy: C.12aarrow_forwardA and B are isomeric dicarbonyl compounds of the molecular formula C5H&O2. The 'H NMR spectrum of A contains a singlet at 2.05 ppm and another singlet at 5.40 ppm. The 'H NMR spectrum of B contains three signals: a singlet at 2.3 ppm, a triplet at 1.10 ppm and a quartet at 2.70 ppm. Suggest structures for A and B and draw them in their respective boxes below. 1st attemptarrow_forwardRank the compounds in each group according to their reactivity towardelectrophilic substitution.(a) Chlorobenzene, o-dichlorobenzene, benzene(b) p-Bromonitrobenzene , nitrobenzene, phenol(c) Fluorobenzene, benzaldehyde, a-xylene(d) Benzonitrile, p-methylbenzonitr ile,p-methoxybenzonitrilearrow_forward
- 8. (a) Benzene derivatives exhibit medium to strong absorption in UV-region. Explain why aniline and phenoxide ion have strong UV-absorptions.arrow_forward11. See Fundamentals P167 for Figure 5.6 Use Figure 5.6 to rank the compounds in each of the following groups in order of their reactivity toward electrophilic aromatic substitution: (a) Nitrobenzene, phenol (hydroxybenzene), toluene (b) Phenol, benzene, chlorobenzene, benzoic acid (c) Benzene, bromobenzene, benzaldehyde, aniline (aminobenzene)arrow_forward(c) A tri-substituted benzene possessing one bromine and two methoxy substituents exhibit three aromatic resonances at 8 6.40, 6.46, and 7.41 ppm. Identify the substitution pattern.arrow_forward
- Compound F may be synthesised by the method attached: When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forwardAn unknown compound has a molecular formula of C3H6O2. Its IR spectrum shows a very strong and broad band at 2980 and a strong sharp peak at 1716 cm-1. It exhibits the following signals in its 1H NMR spectrum (ppm): 1.21 (triplet, 3H), 2.48 (quartet, 2H), 11.7 (singlet, 1H); and the following signals in its 13C NMR spectrum (ppm): 8.9, 27.6, 181.5. Draw the structure of the unknown compound.arrow_forwardA compound of formula C6H10O2 shows only two absorptions in the proton NMR: a singlet at 2.67 ppm and a singlet at2.15 ppm. These absorptions have areas in the ratio 2:3. The IR spectrum shows a strong absorption at 1708 cm-1. Proposea structure for this compound.arrow_forward
- (2) Compound B has a formula CSH100. 1H NMR: 6 3.5 ppm (4H, singlet), & 0.9 ppm (6H, singlet). 13C NMR: 6 64, 41, 12 ppm. IR: no absorption at 1700 cm1. What is the structure of B?arrow_forward3- Identify the aromatic compounds and provide justification for your answers: & H N-arrow_forwardDeduce the structures of compounds A and B, two of the major components of jasmine oil, from the given data. Compound A: C9H10O2; IR absorptions at 3091–2895 and 1743 cm-1; 1H NMR signals at 2.06 (singlet, 3 H), 5.08 (singlet, 2 H), and 7.33 (broad singlet, 5 H) ppm. Compound B: C14H12O2; IR absorptions at 3091–2953 and 1718 cm-1; 1H NMR signals at 5.35 (singlet, 2 H) and 7.26–8.15 (multiplets, 10 H) ppm.arrow_forward
