
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.2, Problem 14.1P
Calculate the nominal mass of each ion. Unless otherwise indicated, use the mass of the most abundant isotope of each element.
(a) 
(b) 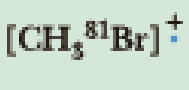
(c) 
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Determine the charge of each ion.(a) oxygen ion with 10 electrons(b) aluminum ion with 10 electrons(c) titanium ion with 18 electrons(d) iodine ion with 54 electrons
State the number of neutrons in an atom of each of the following isotopes. (a) hydrogen-1 (b) carbon-13 (c) cobalt-59 (d) iodine-127
Explain the important distinctions between eachpair of terms:(a) cathode rays and X-rays(b) protons and neutrons(c) nuclear charge and ionic charge(d) periods and groups of the periodic table(e) metal and nonmetal(f) the Avogadro constant and the mole
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Write the electron configurations far each of the following elements: (a) Sc. (b) Ti. (c) Cr. (d) Fe. (e) Ru
Chemistry by OpenStax (2015-05-04)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The percent by mass of nitrogen is 46.7% for a species containing only nitrogen and oxygen. Which of the following could he this species? mg src=Images/HTML_99425-8-23ALQ_image001.jpg alt="" align="top"/>arrow_forwardThe percentage of oxygen by weight in Al2(SO4)3 atomic weights: Al=27, S=32, O=16 is approximately: a. 19. b. 21. c. 56. d. 92.arrow_forwardVanadium (V) and oxygen (O) form a series of compounds with the following compositions: What are the relative numbers of atoms of oxygen in the compounds for a given mass of vanadium?arrow_forward
- What is meant by stating that the charge of an electron is 1? What is the symbol of the electron?arrow_forwardThe element lanthanum has two stable isotopes, lanthanum 138 with an atomic mass of 137.9071u and, lanthanum 139 with an atomic mass of 138.9063u. From atomic mass of La, 138.9u what conclusion can you make about the relative percentage abundance of the isotopes?arrow_forwardWrite a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.(a) the alkali metal with 11 protons and a mass number of 23(b) the noble gas element with 75 neutrons in its nucleus and 54 electrons in the neutral atom(c) the isotope with 33 protons and 40 neutrons in its nucleus(d) the alkaline earth metal with 88 electrons and 138 neutronsarrow_forward
- Magnesium has three isotopes with mass numbers 24, 25, and 26. (a) Write the complete chemical symbol (superscript andsubscript) for each. (b) How many neutrons are in an atom of each isotope?arrow_forwardWrite a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.(a) the chalcogen with a mass number of 125(b) the halogen whose longest-lived isotope is radioactive(c) the noble gas, used in lighting, with 10 electrons and 10 neutrons(d) the lightest alkali metal with three neutronsarrow_forwardWrite the elemental symbol for each of the following atoms and calculate the number of neutrons: (a) atomic number 55, and mass number 133 (b) atomic number 15, mass number 31arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY