
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.7C, Problem 12.3P
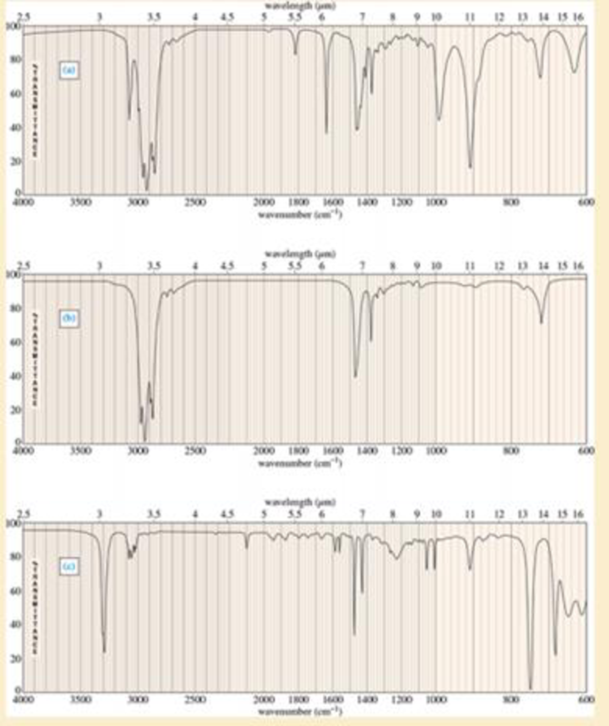
For each hydrocarbon spectrum, determine whether the compound is an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
molecular formula: C5H9N
1. Given the molecular formula, label important peaks on the spectrum and explain how you determined the Structural Formula for your assigned compound.
Please interpet this spectrum as far as the major peaks. For example, is the 3438.78 an NH peak or C-O stretch? What about the other main peaks and also the subsitution shown.
Based on the spectra you located, does your molecule have a carbonyl? If so, what functional group is it a part of (carboxylic acid, ketone, aldehyde, ester, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a carbonyl?
Does your molecule have either an –O-H or –N-H bond? If so, what functional group is it a part of (carboxylic acid, alcohol, amine, amide) and what is the frequency (in wavenumbers) of the absorption peak? If not, what are the approximate frequency ranges for an –O-H and an –N-H bond?
Does your molecule have either an alkyne or nitrile functional group? If so, which functional group is it and what is the frequency (in wavenumbers) of the absorption peak? If not, what is the approximate frequency range for a triple bond?
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Analyze the spectra below assign/ label significant peaks and draw the isomers (correct structure of the molecular formula C5H8Oarrow_forwardYour classmate needs to decide if the spectrum shown below is a 13CNMR or an 1HNMR spectrum of 2-methyl propane. What is your advice to the student and why?arrow_forwardDetermine the structure for the molecule that most likely produced these spectra.arrow_forward
- Consider the following Proton and Carbon NMR spectra for the same compound, and it contains Chlorine. Decide on a structure which is consistent with the spectral data, and 'assign" both spectra as best as you can. "Assign" = draw structure, label (a,b,c etc) and indicate which peaks belong to which type.arrow_forwardMatch the following functional groups with the associated IR frequency. (all measurements below approximate wavenumbers in cm-¹) Alkene (C-H) Alkane (C-H) Carbonyl (C=O) Alcohol (O-H) 1700 cm^- 3050 cm^-✪ 2900 cm^- 3400 cm^-arrow_forwardFirst picture: use the C13NMR spectrum below to determine the structural formula for the compound of molecular formula C5H10O Second picture: picture: use the HNMR spectrum below to determine the structural formula for the compound of molecular formula C6H12O2arrow_forward
- 8. A strong signal in infrared spectroscopy indicates that a molecule matches the emitted electromagnetic radiation and reports a high transmittance. True False 9. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than the C-C bond in an alkyne. True False 10. The electronegativity difference present in a dipole moment within a bond is directly proportional to the electromagnetic field produced. True Falsearrow_forward3. Look at the two spectra below, one of which is 2-methylcyclohexanol and one of which is 3methylcyclohexene. Which spectrum belongs to which compound? Explain your reasoning. 10- 4000 200 3500 3000 2500 1500 1000 600 Spectrum A 90 20 30 20 10 500 3000 2500 2000 1600 1000 500 Vieters kn1 Spectrum Barrow_forward4. what are the major peaks of the spectrum and what chemical is madearrow_forward
- 3. Attached is the IR for one of the compounds you crystallized in this lab. (a) Is this molecule 2- Naphthol, Acetophenone, Aniline or Vanillin? NAME the compound at the bottom of spectrum box. (b) Please identify and assign the major stretching vibrations. 100 Transmittance (%) 50- 4000 3000 1672 7 1666 6 3184 32 3021 50 2975 57 1598 10 2946 67 1690 10 2864 62 1511 10 2848 82 1455 18 2742 70 1464 32 12 1432 13 1398 52 1389 58 1373 66 1300 7 1266 4 1201 23 1500 Wavenumber (cm-¹) 2000 1172 13 1155 6 1125 24 1031 32 960 84 850 42 827 70 814 60 782 70 733 17 633 27 591 55 555 79 549 79 1000 500arrow_forward6) Which of these spectra contains an alkyne? Explain the reasoning behind your choice and circle the peak(s) that are associated with the alkyne. 100 D 100 4000 4500 1000 2008 2000 HAVENUBR AVENUMBERI 1000 1000 jmm 1900 1000 380 100arrow_forwardLabel the major peaks, especially those those related to fumaric acidarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY