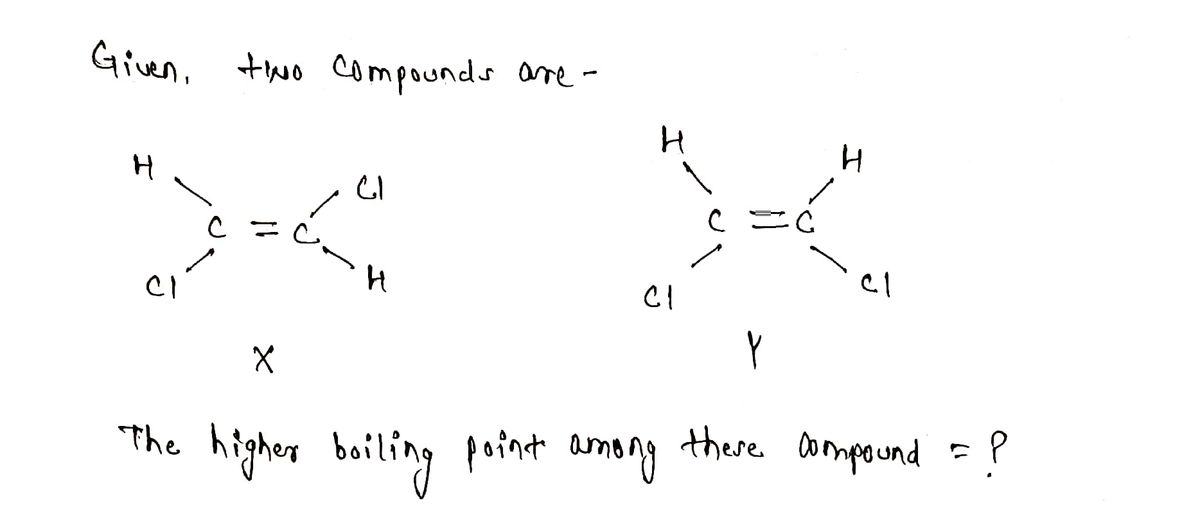
Which compound H CI C=C X has the higher boiling point? Explain briefly. H H C=C Y CI X, because it is more linear. Y, because the dipole moments do not fully cancel. Y, because it is less branched. X, because the dipole moments are far apart.
Q: Find AS for the system, surroundings, and universe when 20.6 g of liquid H₂O is evapourated at 100.0…
A: Given data., Mass of water given = 20.6 gram Molar mass of water = 18.015 g/mol Moles of water =…
Q: Why do we say the two enzyme-catalyzed reactions taking place in your test tube are "coupled"? O…
A: First glucose oxidase produce glconic acid and hydrogen peroxide from glucose. Then these peroxide…
Q: 3. The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling…
A:
Q: A sample of F2 gas has a volume of 5.42L @ STP. How much does it weigh? round to 2 decimal places.
A:
Q: 4. Predict the product of the following reactions. 3-oxobutanoic acid OAC CO₂H aspirin 1. NaBH4 2.…
A:
Q: Thank you. I'm just confused as what is is the reason 122.1 has to be multiplied by (-45543.23)?
A: Answer: This question is based on the understanding of mathematical calculation of the equation.
Q: This is showing as an incorrect answer. Please help. Ty
A:
Q: The major product formed in the following reaction sequence is (1) BH3, THF; H₂O₂/NaOH (2) Ph3P,…
A: Hydroboration-oxidation is a chemical reaction that involves the addition of boron atoms to an…
Q: When 2.41 g of vitamin K is dissolved in 25.0 g of camphor, the freezing point of the solution is…
A: Given depression in freezing point=8.07°C Kf(camphor) =37.8°C/m ∆Tf=iKfm 8.07°C=1 x 37.8°C/m x m m =…
Q: Given the structures provided, write the corresponding IUPAC name (systematic name). & d. xy b. $.…
A: The structures of the given compounds are The IUPAC name of the compounds are
Q: The accepted value of R is 8.314 J mol-¹ K-¹. Which of the following units are exactly equivalent to…
A: Introduction The gas constant, denoted by R, is a fundamental physical constant that appears in many…
Q: How are you getting 3x10^4 because i am getting 3 x 10^-10
A: Characteristics of equilibrium constant are : - If the equation (having equilibrium constant K) is…
Q: steps altogether. Consider a double replacement reaction between Zinc (ll) chloride and sodium…
A: 1.Double displacement reaction are those in which one of the atom in one reactant is replaced by one…
Q: Draw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry.…
A: Given reaction:-
Q: Maple crunch, a new candy, comes in packages of 100.0 g. A 1.00-g sample is burned in a bomb…
A: Answer: This question is based on conservation of energy where energy released due to combustion of…
Q: Complete the balanced neutralization equations for the reactions below. Be sure to include the…
A: An acid reacts with a base to give salt and water. This reaction is called neutralization reaction.…
Q: What sort of reaction is shown below? OH Reduction Acid-Base Esterification Addition Elimination…
A: The reaction between an acid and a base to give salt and water is called neutralization reaction.…
Q: Using the functional groups listed below, indicate the functional group that best describe the…
A: Given : structure of reactant
Q: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the…
A:
Q: Suppose the experiment you are to perform in a given lab period involves substances that are toxic…
A: When conducting laboratory experiments, it is crucial to take the necessary precautions to ensure…
Q: 15.6 Draw the major product for the following pinacol rearrangements. a. b. OH HO OH H₂SO4 H₂SO4
A:
Q: Given the equation 2N₂(g) + O₂(g) = 2N₂O(g) Kc = 1.2x10-¹2, what is Kc for the equation 4N₂(g) +…
A:
Q: Why can we not weigh iodirle on the balance directly? Olodine is flammable, so we need to avoid…
A: To solve this problem we have to know about the property of iodine .
Q: How can you obtain the following compound?
A: Given an unknown enone compound.
Q: Matter can change from one physical state (phase) to another without any change in chemical…
A: For each phase change, determine the sign of ΔPCH and ΔPCS. Use ΔH<0, ΔH>0, ΔS<0 and…
Q: How does one determine the expected Salinity of a water sample via applying constant proportions for…
A: Salinity is a measure of the concentration of dissolved salts in seawater and is a crucial factor in…
Q: Calculate the mass of potassium hydrogen phthalate (KHP), KHC8H4O4 needed to react completely with…
A:
Q: (s) Circle the structures below that are NOT constitutional isomers of C6H100. C6H100 OH fentanyl ОН…
A: Constitutional isomers: The molecular formulas of constitutional isomers are identical, but their…
Q: Consider the two radicals: H3C-CH₂-C-CH3 H Radical A is named: H3C-CH₂-CH₂-CH₂ A Name the two…
A:
Q: What Acid or base could you use to distinguish sodium chloride from sodium iodide? (write the…
A: Sodium chloride formula- NaCl Sidium iodide formula- NaI Here use C. H2SO4 to distinguish between…
Q: For each phase change, determine the sign of ΔPCH and ΔPCS. Use ΔH0, ΔS0. Explain 1. Freezing 2.…
A: For each phase change, determine the sign of ΔPCH and ΔPCS. Use ΔH<0, ΔH>0, ΔS<0 and…
Q: 5. The equilibrium constant Kp for the following reaction is 0.403 at 1000 °C. FeO(s) + CO(g) Fe(s)…
A: Answer: Whether a system is at equilibrium or not, it can be checked by calculating the value of…
Q: How many molecules are in a 7.5L sample of H2 @ STP? Round to 2 decimal places.
A:
Q: When 212. g of benzamide (C,H,NO) are dissolved in 950. g of a certain mystery liquid X, the…
A: Answer: When a non-volatile solute is added in a solvent, it causes a depression in freezing point…
Q: 4. MATCH a structure or term from the following list with each description below. Place the letter…
A: Electrophilic substitution reaction are the reactions in which an electron rich group attacks on the…
Q: Decide which element probably forms a compound with oxygen that has a chemical formula most and…
A:
Q: 2.) Give the product that would be obtained from each of the following Diels-Alder reactions (draw…
A: Diels - Alder reaction is a type of pericyclic reaction in which a diene cyclizes with a dienophile…
Q: Identify the potential coordinating groups in ligands 1 to 5 above and give their denticity i.e.…
A: Denticity: Denticity of the ligand is defined as the number of pair of lone pair donated to Metal…
Q: 3. Fill in the empty boxes with the correct information. Cation Ca2+ NH₂ Anion CI- CIO3 Formula K₂S…
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: 1. 10 mL of 5.0x10 M HCl solution is mixed with 150 mL of water to make a new HCI solution. What is…
A:
Q: 1. Can a set c
A: The quality and reliability of scientific data rely heavily on the accuracy and precision of the…
Q: Consider the reaction: 2SO2(g) + O2(g) → 2SO3(g) ed Text If 279.5 mL of SO₂ reacts with 167.3 mL of…
A:
Q: For O₂ at 25°C and 1 atm calculate: 1. the total translational kinetic energy of O₂ molecules in an…
A: "Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: 2. Determine the structures of the four products (two sub, two elim) that form in this reaction, and…
A: Given that, a reaction scheme is shown below We have to draw the structures of all the products…
Q: 13. Write the formula or name of the compounds below. 6 8 9 1 5 10 2 3 Compound 4 Strontium…
A:
Q: Write the formula for the conjugate acid of each of the following. (Omit states-of-matter from your…
A: Solution - conjugate acid have one more H atom and one more positive charge so we will add one H…
Q: a. Using the VSEPR theory, predict the molecular structure of the following polyatomic ion: PO4³- 3-…
A: VSEPR (Valence Shell Electron Pair Repulsion) theory is used to predict the molecular geometry of a…
Q: Curved arrows are used to illustrate the flow of electrons. Follow the arrows to predict the…
A:
Q: Click on the "draw structure" button to launch the drawing utility. What is the conjugate base of A?…
A: A conjugate base is chemical species that is formed when an acid donates proton. (hydrogen ion ,…
Q: You perform your first titration using your NaOH with a KHP solution. Your KHP solution is made with…
A:

Step by step
Solved in 2 steps with 2 images

- For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name acetic acid dimethyl ether compound hypobromous acid formula or Lewis structure H :0: H-C-C-O-H H H H-C-0-C H H H T HBrO | H Between molecules of the compound? yes O O no O yes no yes hydrogen-bonding force no Between molecules of the compound and molecules of water? O O yes no yes no yes no XThe carbon chlorine bond in chloromethane (CH3Cl) has a bond distance of 178 pm. Assuming a 100% ionic bond, the calculated dipole moment is 8.54 D. The observed (experimental) dipole moment is 1.9 D. What is the percent ionic character of the bond? Enter a whole number percent with two sig figsFormula Lewis Electron Molecular Bond Polar or Attractive Structure Pair Geometry Angle Force Non Polar Geometry Between Molecules CH4 Around First C Around First C C2H4 Around First C Around First C C,H2 Around First C Around First C CH;OH Around O Around O C2H;OH Around O Around O CH20 CH,OCH, Around O Around O CH;COOH Central C Central C НСООН Central C Central C CH;NH2 Around N Around N CH;COCH; Central C Central C
- H- H- H •H C. H- 'N; C H How many pi-bonds are in the molecule shown? I H H H4. The following are valid Lewis structures for CH3SOCH3. Label the formal charges and circle the best Lewis structure. H :ö: H H :0 H a) H-C=S-C-H b) Н—С—S— С-н H. H. H. H. Н :0: Н H :ö: H d) H-C=$-C-H c) Н—С—$—С-н H. H. H HFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name formic acid compound dibromomethane acetic acid formula or Lewis structure :0: || H-C-O-H CH₂Br₂ H :0: | || H-C-C-O-H H Between molecules of the compound? 00 00 yes no yes no yes hydrogen-bonding force no Between molecules of the compound and molecules of water? 00 00 yes no yes no yes no X
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C H H - - chemical symbol, chemical formula or Lewis structure H :0: | || C 1 H | H H | || C-N- | H H - F₂ :NEN 0: :0: - H | C C-O- — H | C I H - H — - H - boiling point (Choose one) ✪ (Choose one) ✪ (Choose one) (Choose one) 09:39 6 00 AWhich of the following bonds are polar? C-O, Cl-Cl, O=O, N-H, C-H.(Electronegativites: C = 2.5, H = 2.1, Cl = 3.0, O = 3.5, N = 3.0). In the selected bonds indicatewhich direction the electron density is greatest.b) Which are polar molecules? ÇI Ö=c=Ö H CH, CH, CH; H,C CH2 CH2 :0. ÇI `H H
- Draw the lewis structure of SO2 ( best resonance) and 2nd best resonance showing the shape and bond angles of each one, the 3D structure with polar bonds or bonds dipole of each one and the molecular polarity. Here is an example of what I want the answer to be like:Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B U H H C - :NEN 0: chemical symbol, chemical formula or Lewis structure H :0: | || C H HIC - H F₁ H :0: | || с C - : 0: H -0. - C N- I H H C-H H 1 C —E H - H boiling point ✓ (Choose one) C 1 (highest) 2 3 4 (lowest) (Choose one) ↑ (Choose one) C olo 18 Ar✔nk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest Bolling puint, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure H H-C-F: H :O: | || HIC-C-F: 1 H H H H :O: | || HIC-C-0-H F₂ boiling point ✓ (Choose one) 1 (highest) 2 34 4 (lowest) (Choose one) ✓ (Choose one) ✓ (Choose one) ✓

