Information: Each spectra below was obtained from a pure compound. Mass Spectrum parent peaks (M) are listed for all examples. IR peaks listed are strong (s) unless otherwise indicated for signals above 1500 cm¹ ¹H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Important coupling constants (/-values) are listed next to the peaks for some examples. For some spectra, an inset (grey box) is also given showing a "zoom-in" on an important part of the spectrum. Mass Spectrometry (not shown): [M] = 158 m/z Infrared Spectroscopy (not shown): 2982, 1731, 1717, 1149 cm² ¹H Nuclear Magnetic Resonance. "C Nuclear Magnetic Resonance. 220 2H 200 180 160 140 3H PPM 120 100 9H 80 8 8 20
Information: Each spectra below was obtained from a pure compound. Mass Spectrum parent peaks (M) are listed for all examples. IR peaks listed are strong (s) unless otherwise indicated for signals above 1500 cm¹ ¹H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Important coupling constants (/-values) are listed next to the peaks for some examples. For some spectra, an inset (grey box) is also given showing a "zoom-in" on an important part of the spectrum. Mass Spectrometry (not shown): [M] = 158 m/z Infrared Spectroscopy (not shown): 2982, 1731, 1717, 1149 cm² ¹H Nuclear Magnetic Resonance. "C Nuclear Magnetic Resonance. 220 2H 200 180 160 140 3H PPM 120 100 9H 80 8 8 20
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 3E
Related questions
Question
determine the chemical structure and name the structure.
![Information: Each spectra below was obtained from a pure compound.
Mass Spectrum parent peaks (M) are listed for all examples. IR peaks listed are strong (s) unless otherwise indicated for signals above 1500 cm¹
¹H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Important coupling constants (/-values) are listed next to
the peaks for some examples. For some spectra, an inset (grey box) is also given showing a "zoom-in" on an important part of the spectrum.
Mass Spectrometry (not shown): [M] = 158 m/z
Infrared Spectroscopy (not shown): 2982, 1731, 1717, 1149 cm³¹
¹H Nuclear Magnetic Resonance.
1³C Nuclear Magnetic Resonance.
220
2H
200
180
160
140
2
3H PPM
120
PPM
100
9H
80
60
40
8
o](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb5f1630e-c465-44f2-a868-b429d99d07e1%2Fc26e9845-e131-4c9b-88ab-6c46b846eeb1%2Fdkv3h88_processed.png&w=3840&q=75)
Transcribed Image Text:Information: Each spectra below was obtained from a pure compound.
Mass Spectrum parent peaks (M) are listed for all examples. IR peaks listed are strong (s) unless otherwise indicated for signals above 1500 cm¹
¹H NMR Spectra, the integral is given in number of hydrogens (#H) or as a relative ratio. Important coupling constants (/-values) are listed next to
the peaks for some examples. For some spectra, an inset (grey box) is also given showing a "zoom-in" on an important part of the spectrum.
Mass Spectrometry (not shown): [M] = 158 m/z
Infrared Spectroscopy (not shown): 2982, 1731, 1717, 1149 cm³¹
¹H Nuclear Magnetic Resonance.
1³C Nuclear Magnetic Resonance.
220
2H
200
180
160
140
2
3H PPM
120
PPM
100
9H
80
60
40
8
o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 6 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
So which way do you write it? Like this or this? Also, what is the name of the structure.
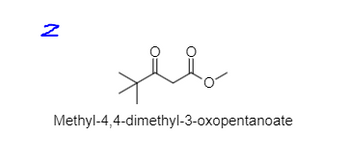
Transcribed Image Text:N
مهلا
Methyl-4,4-dimethyl-3-oxopentanoate
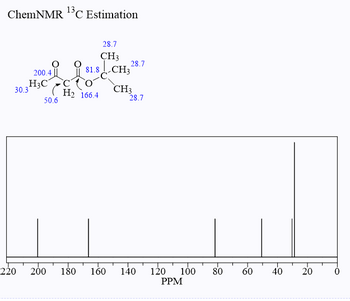
Transcribed Image Text:ChemNMR ¹3³C Estimation
30.3
220
200.4
H3C
50.6
28.7
CH3
81.8-CH328.7
H₂ 166.4
CH3
28.7
200 180 160 140 120 100
PPM
80
60
40
20
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning