Express the molality to two significant figures. molality = 0.66 m NaCl Submit Previous Answers All attempts used; correct answer displayed Note that the molality of a solution is the moles of solute per kilogram of Solving for the molality begins with determining the amount of the solute mass. This value is then divided by the mass of the solvent (in kilogram Part B Calculate the mole fraction of the solution. Express the mole fraction to two significant figures. X = 1.2x10-²
Express the molality to two significant figures. molality = 0.66 m NaCl Submit Previous Answers All attempts used; correct answer displayed Note that the molality of a solution is the moles of solute per kilogram of Solving for the molality begins with determining the amount of the solute mass. This value is then divided by the mass of the solvent (in kilogram Part B Calculate the mole fraction of the solution. Express the mole fraction to two significant figures. X = 1.2x10-²
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.98QE
Related questions
Question
Could I have help with how to get the answer listed for question B?

Transcribed Image Text:MISSED THIS? Watch KCV 13.5. IWE 13.5: Read Section 13.5. You can click
on the Review link to access the section in your e Text.
An aqueous solution contains 3.7 % NaCl by mass.
Express the molality to two significant figures.
molality= 0.66 m NaCl
Submit Previous Answers
All attempts used; correct answer displayed
Note that the molality of a solution is the moles of solute per kilogram of
Solving for the molality begins with determining the amount of the solute
mass. This value is then divided by the mass of the solvent (in kilograms
Part B
Calculate the mole fraction of the solution.
Express the mole fraction to two significant figures.
X= 1.2x10-²
Submit
Previous Answers
Expert Solution
Step 1
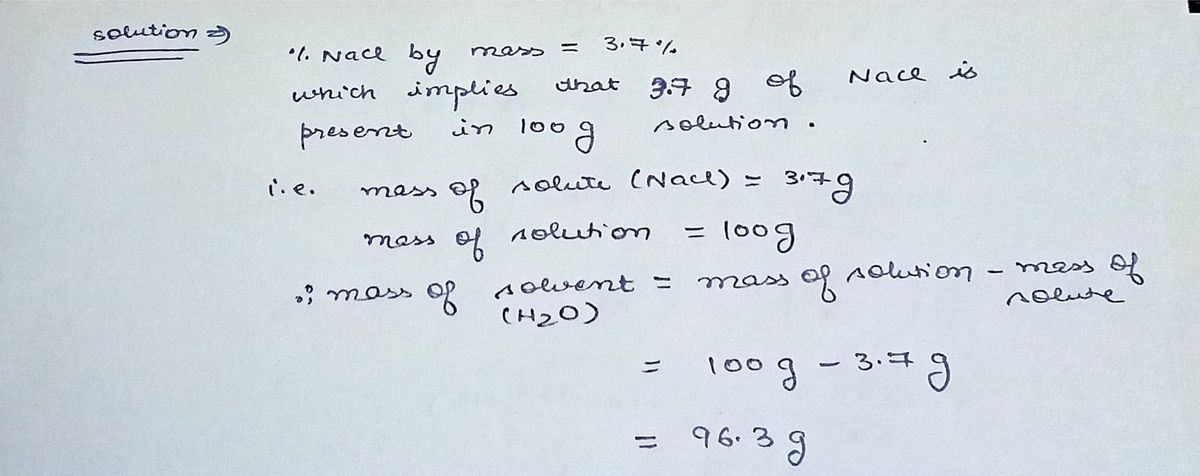
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning