For each part of this question, consider the following scenario: You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50 M. You have a 750.0 mL volumetric flask available to make this stock solution in. You are then tasked with using this stock solution to prepare a sample for use in an experiment. The experimental sample should have a final volume of 5.00 mL and a concentration of 0.150 M Mg(HCO3)2. a) What is the molar mass of Mg(HCO3)2? b) How many moles of the solute would be needed to create the stock solution using the volumetric flask available? c) How many grams of the solute should be added to the volumetric flask to create the stock solution? d) What volume, in mL, of the stock solution should be used to prepare the experimental solution through dilution?
For each part of this question, consider the following scenario: You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50 M. You have a 750.0 mL volumetric flask available to make this stock solution in. You are then tasked with using this stock solution to prepare a sample for use in an experiment. The experimental sample should have a final volume of 5.00 mL and a concentration of 0.150 M Mg(HCO3)2. a) What is the molar mass of Mg(HCO3)2? b) How many moles of the solute would be needed to create the stock solution using the volumetric flask available? c) How many grams of the solute should be added to the volumetric flask to create the stock solution? d) What volume, in mL, of the stock solution should be used to prepare the experimental solution through dilution?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 40P
Related questions
Question

Transcribed Image Text:For each part of this question, consider the following scenario:
You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50
M. You have a 750.0 mL volumetric flask available to make this stock solution in.
You are then tasked with using this stock solution to prepare a sample for use in an
experiment. The experimental sample should have a final volume of 5.00 mL and a
concentration of 0.150 M Mg(HCO3)2.
a) What is the molar mass of Mg(HCO3)2?
b) How many moles of the solute would be needed to create the stock solution using
the volumetric flask available?
c) How many grams of the solute should be added to the volumetric flask to create
the stock solution?
d) What volume, in mL, of the stock solution should be used to prepare the
experimental solution through dilution?
Expert Solution
Step 1
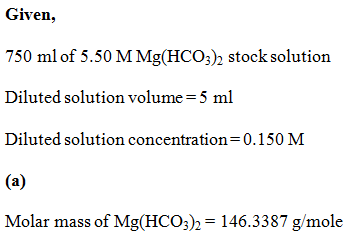
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning