An acid (HA) dissociates as follows: HA - H* +A minus Arrow should be interpreted as an equilibrium arrow. The pH of the 0.26 M solution of HA is 4.90. What is [H*]? Express your answer as a decimal, not an exponent. Please include a proper (abbreviated) unit.
An acid (HA) dissociates as follows: HA - H* +A minus Arrow should be interpreted as an equilibrium arrow. The pH of the 0.26 M solution of HA is 4.90. What is [H*]? Express your answer as a decimal, not an exponent. Please include a proper (abbreviated) unit.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.87QE
Related questions
Question
2.
![An acid (HA) dissociates as follows:
HA - H* +A minus
Arrow should be interpreted as an equilibrium arrow.
The pH of the 0.26 M solution of HA is 4.90.
What is [H*]?
Express your answer as a decimal, not an exponent.
Please include a proper (abbreviated) unit.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6746fa6f-1856-443b-addc-ecae5d9da9b9%2F65d06806-5435-47a6-98ae-cce70cf8fcc5%2Fohbwvoe.jpeg&w=3840&q=75)
Transcribed Image Text:An acid (HA) dissociates as follows:
HA - H* +A minus
Arrow should be interpreted as an equilibrium arrow.
The pH of the 0.26 M solution of HA is 4.90.
What is [H*]?
Express your answer as a decimal, not an exponent.
Please include a proper (abbreviated) unit.
Expert Solution
Step 1
Since,
Step 2
Given,
pH of the weak acid=4.90
[H+]=?
Use the above formula to get [H+].
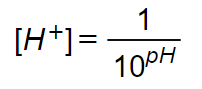
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning