A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: 190. 170. temperature (°C) 150. 130. 110. 0. 10. What is the boiling point of X ? Use this graph to answer the following questions: heat added (kJ/mol) What phase (physical state) of X would you expect to find in the flask after 11 kJ/mol of heat has been added? 20. 0 0 0 % °℃ (check all that apply) solid liquid 30. gas 40. ? olo 18 Ar
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: 190. 170. temperature (°C) 150. 130. 110. 0. 10. What is the boiling point of X ? Use this graph to answer the following questions: heat added (kJ/mol) What phase (physical state) of X would you expect to find in the flask after 11 kJ/mol of heat has been added? 20. 0 0 0 % °℃ (check all that apply) solid liquid 30. gas 40. ? olo 18 Ar
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Thank you for taking the time to provide feedback! Please let us know more here so we will not miss it. We have credited a question to your account.
Your Question:
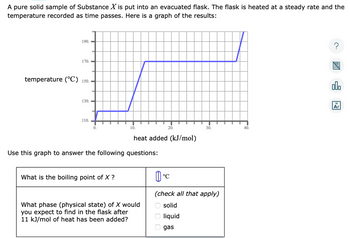
Transcribed Image Text:A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the
temperature recorded as time passes. Here is a graph of the results:
190.
170.
temperature (°C) 150.
130.
110.
0.
10.
What is the boiling point of X ?
Use this graph to answer the following questions:
heat added (kJ/mol)
What phase (physical state) of X would
you expect to find in the flask after
11 kJ/mol of heat has been added?
20.
0 0 0
%
°℃
(check all that apply)
solid
liquid
30.
gas
40.
?
olo
18
Ar
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
