3. Calculate the mass percent of NaHCO3 based on the manufacturer's list of ingredients: 325 mg aspirin, 1000 mg citric acid, 1916 mg NaHCO,. a. Show your calculation for the mass percent of NaHCO3. b. Compare this value with your average mass percent of NaHCO, in Alka-Seltzer. How close was your experimental value to the mass percent calculated from the ingredients? Explain possible sources of error that could explain any differences.
3. Calculate the mass percent of NaHCO3 based on the manufacturer's list of ingredients: 325 mg aspirin, 1000 mg citric acid, 1916 mg NaHCO,. a. Show your calculation for the mass percent of NaHCO3. b. Compare this value with your average mass percent of NaHCO, in Alka-Seltzer. How close was your experimental value to the mass percent calculated from the ingredients? Explain possible sources of error that could explain any differences.
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts need more information to provide you with a solution. For b The experimental value is not available for comparison , kindly provide Please resubmit your question, making sure it's detailed and complete. We've credited a question to your account.
Your Question:
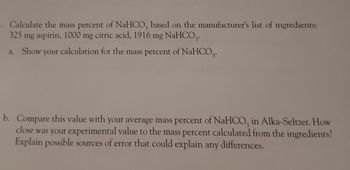
Transcribed Image Text:3. Calculate the mass percent of NaHCO3 based on the manufacturer's list of ingredients:
325 mg aspirin, 1000 mg citric acid, 1916 mg NaHCO,.
a. Show your calculation for the mass percent of NaHCO3.
b. Compare this value with your average mass percent of NaHCO, in Alka-Seltzer. How
close was your experimental value to the mass percent calculated from the ingredients?
Explain possible sources of error that could explain any differences.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
