1. Summarize the experimental data obtained in this experiment in the table below: Percent Recovery from Recrystallization (%) Compound Salicylic Acid Crude Yield Purified (g) Yield (g) 16 7.10 7.63 8.10 12.05 15.12 2.2065 Chemical Shift (ppm) 14 1.6759 2. In the saponification reaction performed in this experiment, the procedure calls for the addition of 15mL 5.0M NaOH solution and 1.50g methyl salicylate as reactants. Determine the volume of 2.0M H₂SO, required to exactly neutralize this solution. Referring to the experimental procedure, how does this compare with the volume used to reach neutralization? 1H 2H 1H 1H 1H 3. Using the experimentally obtained 'HNMR spectrum of salicylic acid shown below, complete the following table. 12 Integration 10 4 76 O 8 PPM 8 OH OH Percent Yield (%) Splitting 112 6 MP of purified material (°C) 163-164 4 2 Assignment 0
1. Summarize the experimental data obtained in this experiment in the table below: Percent Recovery from Recrystallization (%) Compound Salicylic Acid Crude Yield Purified (g) Yield (g) 16 7.10 7.63 8.10 12.05 15.12 2.2065 Chemical Shift (ppm) 14 1.6759 2. In the saponification reaction performed in this experiment, the procedure calls for the addition of 15mL 5.0M NaOH solution and 1.50g methyl salicylate as reactants. Determine the volume of 2.0M H₂SO, required to exactly neutralize this solution. Referring to the experimental procedure, how does this compare with the volume used to reach neutralization? 1H 2H 1H 1H 1H 3. Using the experimentally obtained 'HNMR spectrum of salicylic acid shown below, complete the following table. 12 Integration 10 4 76 O 8 PPM 8 OH OH Percent Yield (%) Splitting 112 6 MP of purified material (°C) 163-164 4 2 Assignment 0
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Hi and thanks for your question! Unfortunately, your question violates our terms of use. Try rephrasing your question or asking a new one below. We've credited a question back to your account. Apologies for the inconvenience.
Your Question:
Only qno3 solve full accurate with diagrams follow all instructions of qno3. ok
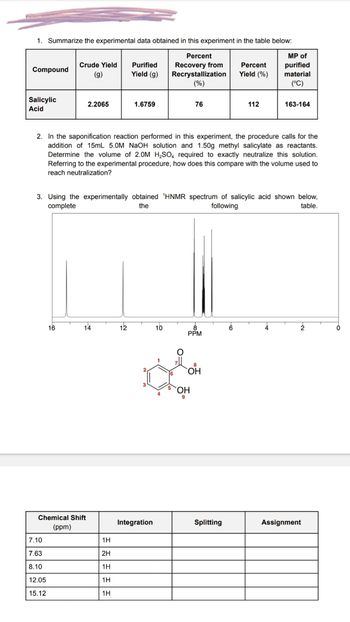
Transcribed Image Text:1. Summarize the experimental data obtained in this experiment in the table below:
Percent
Recovery from
Recrystallization
(%)
Compound
Salicylic
Acid
Crude Yield Purified
(g)
Yield (g)
16
7.10
7.63
8.10
12.05
15.12
2.2065
Chemical Shift
(ppm)
14
1.6759
2. In the saponification reaction performed in this experiment, the procedure calls for the
addition of 15mL 5.0M NaOH solution and 1.50g methyl salicylate as reactants.
Determine the volume of 2.0M H₂SO, required to exactly neutralize this solution.
Referring to the experimental procedure, how does this compare with the volume used to
reach neutralization?
1H
2H
1H
1H
1H
3. Using the experimentally obtained 'HNMR spectrum of salicylic acid shown below,
complete
the
following
table.
12
Integration
10
4
76
O
8
PPM
8
OH
OH
Percent
Yield (%)
Splitting
112
6
MP of
purified
material
(°C)
163-164
4
2
Assignment
0
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
