0.8 g of a gas fills up a flask of 850 mL at 183.5 torr and 30˚C. The empirical formula of the gas is CHCl. What is the molecular formula of the gas?
0.8 g of a gas fills up a flask of 850 mL at 183.5 torr and 30˚C. The empirical formula of the gas is CHCl. What is the molecular formula of the gas?
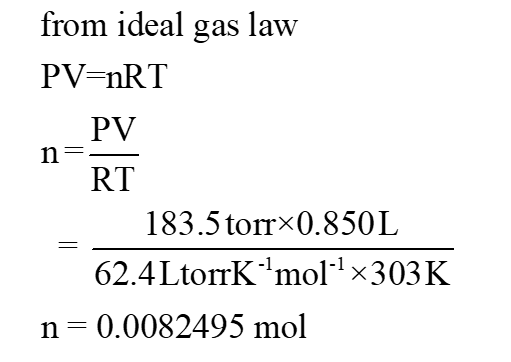
The expression of ideal gas law is shown below:
PV = nRT
Where,
P = pressure of the ideal gas
V = volume of the container
n = number of moles of gas
R = gas constant
T = temperature
Given information:
Pressure of gas = 183.5 torr
Volume of the flask = 850 mL
Temperature = 300 C
Given mass of the gas = 0.8 g
The empirical formula of the gas = CHCl
Empirical molecular weight = molar mass of C + molar mass of H + molar mass of Cl
=12+1+35.5
= 48.5
number of moles of gas is calculated as shown below:
Step by step
Solved in 7 steps with 3 images









